Abstract
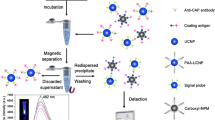
We report on a novel immunoassay for porcine pseudorabies virus (PRV) antibody that is based on fluorescence signal amplification induced by silver(I) ion exchange in CdSe nanocrystals. An antigen-antibody-secondary antibody sandwich structure was first formed from PRV, PRV antibody, and CdSe-labeled rabbit anti-pig antibody. Then, the Cd(II) ions in the CdSe labels were released by a cation exchange reaction with Ag(I). Released Cd(II) was finally quantified using the sensitive fluorescent probe Rhodamine 5 N. Due to this signal amplification, the sensitivity and linear range of the immunoassay were largely improved (compared to the traditional ELISA) in having a limit of detection as low as 1.2 ng mL−1 of PRV antibody and a linear range from 2.44 to 312 ng mL−1. The successful determination of PRV antibody in pig serum samples is proof for the utility of the method.

A simple, rapid and sensitive method for the detection of PRV antibody through the fluorescence signal amplification caused by cation-exchange in CdSe NCs was reported. The CdSe NCs labeled rabbit anti-pig IgG was used to capture the PRV antibody. After the immunoreaction, the Cd2+ in the CdSe labels was completely replaced by the cation-exchange reaction with Ag+. Then Cd2+sensitive fluorescence indicator Rhod-5 N was added to bind with Cd2+ and caused the fluorescence signal enhance substantially. Thus a novel method for rapid and sensitive detection of porcine pseudorabies based on the fluorescence signal amplification was developed.






Similar content being viewed by others
References
Baskerville A, McFerran J, Dow C (1973) Aujeszky’s disease in pigs. Vet Bull 43:456480
Zanin E, Capua I, Casaccia C, Zuin A, Moresco A (1997) Isolation and characterization of Aujeszky’s disease virus in captive brown bears from Italy. J Wildl Dis 33:632634
Iglesias G, Pijoan C, Molitor T (1992) Effects of pseudorabies virus infection upon cytotoxicity and antiviral activities of porcine alveolar macrophages. Comp Immunol Microbiol Infect Dis 15:249259
Iglesias G, Pijoan C, Molitor T (1989) Interactions of pseudorabies virus with swine alveolar macrophages: Effects of virus infection on cell functions. J Leukoc Biol 45:410415
Bolin S, Bolin C (1984) Pseudorabies virus infection of six-and ten-day-old porcine embryos. Theriogenology 22:101108
Muller T, Hahn E, Tottewitz F, Kramer M, Klupp B, Mettenleiter T, Freuling C (2011) Pseudorabies virus in wild swine: a global perspective. Arch Virol 156:115
Ruiz-Fons F, Segales J, Gortazar C (2008) A review of viral diseases of the European wild boar: effects of population dynamics and reservoir role. Vet J 176:158169
Boadella M, Gortazar C, Vicente J, Ruiz-Fons F (2012) Wild boar: an increasing concern for Aujeszky’s disease control in pigs? BMC Vet Res 8:7
Ostrowski M, Galeota J, Jar A, Platt K, Osorio FA, Lopez O (2002) Identification of neutralizing and nonneutralizing epitopes in the porcine reproductive and respiratory syndrome virus GP5 ectodomain. J Virol 76:42414250
He Q, Velumani S, Du Q, Lim CW, Ng FK, Donis R, Kwang J (2007) Detection of H5 avian influenza viruses by antigen-capture enzyme-linked immunosorbent assay using H5-specific monoclonal antibody. Clin Vaccine Immunol 14:617623
Xu XG, Chen GD, Huang Y, Ding L, Li ZC, Chang CD, Wang CY, Tong DW, Liu HJ (2012) Development of multiplex PCR for simultaneous detection of six swine DNA and RNA viruses. J Virol Methods 183:6974
Yue F, Cui S, Zhang C, Yoon KJ (2009) A multiplex PCR for rapid and simultaneous detection of porcine circovirus type 2, porcine parvovirus, porcine pseudorabies virus, and porcine reproductive and respiratory syndrome virus in clinical specimens. Virus genes 38:392397
Dong H, Li CM, Chen W, Zhou Q, Zeng ZX, Luong JHT (2006) Sensitive amperometric immunosensing using polypyrrolepropylic acid films for biomolecule immobilization. Anal Chem 78:74247431
Wilson MS, Nie W (2006) Electrochemical multianalyte immunoassays using an array-based sensor. Anal Chem 78:25072513
Yin Z, Liu Y, Jiang LP, Zhu JJ (2011) Electrochemical immunosensor of tumor necrosis factor α based on alkaline phosphatase functionalized nanospheres. Biosens Bioelectron 26:18901894
Zhao H, Sheng Q, Zheng J (2012) Direct electrochemistry and electrocatalysis of horseradish peroxidase on a gold electrode modified with a polystyrene and multiwalled carbon nanotube composite film. Microchim Acta 176:18
Ambrosi A, AiroF MA (2009) Ehanced gold nanoparticle based ELISA for a breast cancer biomaker. Anal Chem 82:11511156
Manclús JJ, Abad A, Lebedev MY, Mojarrad F, Micková B, Mercader JV, Primo J, Miranda MA, Montoya A (2004) Development of a monoclonal immunoassay selective for chlorinated cyclodiene insecticides. J Agric Food Chem 52:27762784
Kuhlmann W, Peschke P (1986) Glucose oxidase as label in histological immunoassays with enzyme-amplification in a two-step technique: coimmobilized horseradish peroxidase as secondary system enzyme for chromogen oxidation. Histochem Cell Biol 85:1317
Suzuki A, Itoh F, Hinoda Y, Imai K (1995) Double determinant immunopolymerase chain reaction: a sensitive method for detecting circulating antigens in human sera. Cancer Sci 86:885889
Yazynina EV, Zherdev AV, Dzantiev BB, Izumrudov VA, Gee SJ, Hammock BD (1999) Immunoassay techniques for detection of the herbicide simazine based on use of oppositely charged water-soluble polyelectrolytes. Anal Chem 71:35383543
Chen Y, Ren H, Liu N, Sai N, Liu X, Liu Z, Gao Z, Ning B (2010) A fluoroimmunoassay based on quantum dot-streptavidin conjugate for the detection of chlorpyrifos. J Agric Food Chem 58:88958903
Luo Z, Chen K, Lu D, Han H, Zou M (2011) Synthesis of p-aminothiophenol-embedded gold/silver core-shell nanostructures as novel SERS tags for biosensing applications. Microchim Acta 173:149156
Ma C, Xie G, Zhang W, Liang M, Liu B, Xiang H (2012) Label-free sandwich type of immunosensor for hepatitis C virus core antigen based on the use of gold nanoparticles on a nanostructured metal oxide surface. Microchim Acta 178:110
Tian J, Huang J, Zhao Y, Zhao S (2012) Electrochemical immunosensor for prostate-specific antigen using a glassy carbon electrode modified with a nanocomposite containing gold nanoparticles supported with starch-functionalized multi-walled carbon nanotubes. Microchim Acta 178:18
Li J, Zhang T, Ge J, Yin Y, Zhong W (2009) Fluorescence signal amplification by cation exchange in ionic nanocrystals. Angew Chem Int Ed 48:15881591
Yao J, Schachermeyer S, Yin Y, Zhong W (2011) Cation exchange in ZnSe nanocrystals for signal amplification in bioassays. Anal Chem 83:402408
Sheng Z, Hu D, Zhang P, Gong P, Gao D, Liu S, Cai L (2012) Cation exchange in aptamer conjugated CdSe nanoclusters: a novel fluorescence signal amplification for cancer cell detection. Chem Commun 48:42024204
Hu D, Han H, Zhou R, Dong F, Bei W, Jia F, Chen H (2008) Gold (III) enhanced chemiluminescence immunoassay for detection of antibody against ApxIV of Actinobacillus pleuropneumoniae. Analyst 133:768773
Sheng Z, Han H, Hu D, Liang J, He Q, Jin M, Zhou R, Chen H (2009) Quantum dots-gold (III)-based indirect fluorescence immunoassay for high-throughput screening of APP. Chem Commun 45:25592561
Qian J, Zhang C, Cao X, Liu S (2010) Versatile immunosensor using a quantum dot coated silica nanosphere as a label for signal amplification. Anal Chem 82:64226429
Qu L, Peng X (2002) Control of photoluminescence properties of CdSe nanocrystals in growth. J Am Chem Soc 124:20492055
Gao J, Chen K, Xie R, Xie J, Lee S, Cheng Z, Peng X, Chen X (2010) Ultrasmall near-infrared non-cadmium quantum dots for in vivo tumor imaging. Small 6:256261
Breus VV, Heyes CD, Nienhaus GU (2007) Quenching of CdSe-ZnS core-shell quantum dot luminescence by water-soluble thiolated ligands. J Phys Chem C 111:1858918594
Ipe BI, Shukla A, Lu H, Zou B, Rehage H, Niemeyer CM (2006) Dynamic light scattering analysis of the electrostatic interaction of hexahistidine tagged cytochrome P450 enzyme with semiconductor quantum dots. Chem Phys Chem 7:11121118
Soibinet M, Souchon V, Leray I, Valeur B (2008) Rhod-5 N as a fluorescent molecular sensor of cadmium (II) ion. J Fluoresc 18:10771082
Son DH, Hughes SM, Yin Y, Alivisatos AP (2004) Cation exchange reactions in ionic nanocrystals. Science 306:10091012
Ribou AC, Salmon JM, Vigo J, Goyet C (2007) Measurements of calcium with a fluorescent probe Rhod-5 N: Influence of high ionic strength and pH. Talanta 71:437442
Yao J, Han X, Zeng S, Zhong W (2012) Detection of femtomolar proteins by nonfluorescent ZnS nanocrystal clusters. Anal Chem 84:1645–1652
Cowles CL, Zhu X, Publicover NG (2011) Fluorescence signal transduction mechanism for immunoassay based on zinc ion release from ZnS nanocrystals. Analyst 136:2975–2980
Acknowledgements
The authors gratefully acknowledge the support for this research from National Natural Science Foundation of China (20975042, 21175051), the Fundamental Research Funds for the Central Universities (2010PY009, 2011PY139) and the Natural Science Foundation of Hubei Province Innovation Team (2011CDA115).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 333 kb)
Rights and permissions
About this article
Cite this article
Li, X., Chen, K., Huang, L. et al. Sensitive immunoassay for porcine pseudorabies antibody based on fluorescence signal amplification induced by cation exchange in CdSe nanocrystals. Microchim Acta 180, 303–310 (2013). https://doi.org/10.1007/s00604-012-0934-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-012-0934-y