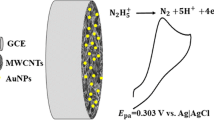
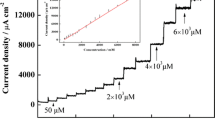
Abstract
Multiwalled carbon nanotubes with nanosized sputtered gold were used to modify a glassy carbon electrode (GCE). The substrate was characterized by scanning electron microscopy (SEM), X-ray diffraction, cyclic voltammetry and amperometry. SEM micrographs indicated an uniform coverage of the carbon nanotubes with nanosized (poly)crystalline gold. Cyclic voltammetry reveals that peak separation of the unmodified GCE in the presence of 1 mM ferricyanide is 131 mV, but 60 mV only for the modified GCE. In addition, the oxidation of NADH (1 mmol L−1 solution) begins at negative potentials (around −100 mV vs. Ag/AgCl), and the anodic peak potential (corresponding to the irreversible oxidation of NADH) is found at +94 mV. The effect of pH on the electrocatalytic activity was studied in the range from 5.4 to 8.0. The relationship between the anodic peak potential and the pH indicated a variation of −33.5 mV/pH which is in agreement with a two-electron and one-proton reaction mechanism. Amperometry, performed at either −50 or +50 mV vs. an Ag/AgCl reference electrode, indicates that the modified electrode is a viable amperometric sensor for NADH. At a working potential of +50 mV, the response to NADH is linear in the concentration range from 1 to 100 μmol L−1, with an RSD of 6% (n = 4).

Multiwalled carbon nanotubes with nanosized sputtered gold were used to modify a glassy carbon electrode. The oxidation of NADH (1 mmol L−1) begins at negative potentials (around −100 mV vs. Ag/AgCl), and the anodic peak potential (corresponding to the irreversible oxidation of NADH) is found at +94 mV.



Similar content being viewed by others
References
Vashist SK, Zheng D, Al-Rubeaan K, Luong JHT, Sheu FS (2011) Advances in carbon nanotube based electrochemical sensors for bioanalytical applications. Biotechnol Adv 29:169–188
Wang J (2005) Carbon-nanotube based electrochemical biosensors: A review. Electroanalysis 17:7–14
Musameh M, Lawrence NS, Wang J (2005) Electrochemical activation of carbon nanotubes. Electrochem Commun 7:14–18
Clark WM (1972) Oxidation–reduction potentials of organic system. R.E. Krieger Publishing, Huntington
Dixon M, Webb EC (1979) Enzymes. Longman, London
Rahman MM, Shiddiky MJA, MdA R, Shim Y-B (2009) A lactate biosensor based on lactate dehydrogenase/nictotinamide adenine dinucleotide (oxidized form) immobilized on a conducting polymer/multiwall carbon nanotube composite film. Anal Biochem 384:159–165
Deng L, WangY SL, Wen D, Wang F, Dong S (2008) A sensitive NADH and glucose biosensor tuned by visible light based on thionine bridged carbon nanotubes and gold nanoparticles multilayer. Biosens Bioelectron 24:951–957
Meng L, Wu P, Chen G, Cai C, Sun Y, Yuan Z (2009) Low potential detection of glutamate based on the electrocatalytic oxidation of NADH at thionine/single-walled carbon nanotubes composite modified electrode. Biosens Bioelectron 24:1751–1756
Hart P, Serban S, Jones LJ, Biddle N, Pittson R, Drago GA (2006) Selective and rapid biosensor integrated into a commercial hand-held instrument for the measurement of ammonium ion in sewage effluent. Anal Lett 39:1657–1667
Antiochia R, Gorton L (2007) Development of a carbon nanotube paste electrode osmium polymer-mediated biosensor for determination of glucose in alcoholic beverages. Biosens Bioelectron 22:2611–2617
Lupu A, Compagnone D, Palleschi G (2004) Screen-printed enzyme electrodes for the detection of marker analytes during wine-making. Anal Chim Acta 513:67–72
Avramescu A, Noguer T, Avramescu M, Marty JL (2002) Screen-printed biosensors for the control of wine quality based on lactate and acetaldehyde determination. Anal Chim Acta 458:203–213
Radoi A, Compagnone D, Batič M, Klinčar J, Gorton L, Palleschi G (2007) NADH screenprinted electrodes modified with zirconium phosphate Meldola Blue and Reinecke salt. Application to the detection of glycerol by FIA. Anal Bioanal Chem 387:1049–1058
Pereira AC, Aguiar MR, Kisner A, Macedo DV, Kubota LT (2007) Amperometric biosensor for lactate based on lactate dehydrogenase and Meldola Blue coimmobilized on multi-wall carbon-nanotube. Sensor Actuator B Chem 124:269–276
Tsai YC, Tsai MC, Chiu CC (2007) Amperometric ethanol biosensor based on poly (vinyl alcohol) multiwalled carbon nanotube–alcohol dehydrogenase biocomposite. Biosens Bioelectron 22:3051–3056
Jena BK, Raj CR (2006) Electrochemical biosensor based on integrated assembly of dehydrogenase enzymes and gold nanoparticles. Anal Chem 78:6332–6339
Ozkar S (2009) Enhancement of catalytic activity by increasing surface area in heterogeneous catalysis. Appl Surf Sci 256:1272–1277
Pool R (1990) Clusters: strange morsels of matter. Science 248:1186–1188
Gorton L (2002) Electrochemistry of NAD(P)+/NAD(P)H. In: Bard AJ, Stratmann M (eds) Encyclopedia of electrochemistry. Wiley-VCH, Weinheim, pp 67–143
Rodkey FL (1955) Oxidation–reduction potentials of the diphosphopyridine nucleotide system. J Biol Chem 213:777–786
Moiroux J, Elving PJ (1978) Effect of adsorbtion, electrode material, operational variables on the oxidation dihydronicotinamide adenine dinucleotide at carbon electrodes. Anal Chem 50:1056–1062
Samec Z, Elving PJ (1983) Anodic oxidation of dihydronicotinamide adenine dinucleotide at solid electrodes; mediation by surface species. J Electroanal Chem 144:217–234
Blaedel WJ, Jenkins RA (1975) Electrochemical oxidation of reduced nicotinammide adenine dinucleotide. Anal Chem 47:1337–1343
Gorton L, Domınguez E (2002) Electrocatalytic oxidation of NAD(P)H at mediator modified electrodes. Rev Mol Biotechnol 82:371–392
Dilgin DG, Gligor D, İsmet Gökçel H, Dursun Z, Dilgin Y (2011) Glassy carbon electrode modified with poly-Neutral Red for photoelectrocatalytic oxidation of NADH. Microchim Acta 173:469–476
Agui L, Yanez-Sedeno P, Pingarron JM (2008) Role of carbon nanotubes in electroanalytical chemistry: a review. Anal Chim Acta 622:11–47
Merkoci A (2006) Carbon nanotube in analytical sciences. Microchim Acta 152:157–174
Jacobs CB, Peairs MJ, Venton BJ (2010) Carbon nanotubes based electrochemical sensors for biomolecules. Anal Chim Acta 662:105–127
Wang Y, You C, Zhang S, Kong J, Marty J-L, Zhao D, Liu B (2009) Electrocatalytic oxidation of NADH at mesoporous carbon modified electrodes. Microchim Acta 167:75–79
Vastarella W, Nicastri R (2005) Enzyme/semiconductor nanoclusters combined systems for novel amperometric biosensors. Talanta 66:627–633
Curulli A, Valentini F, Padeletti G, Viticoli M, Caschera D, Palleschi G (2005) Gold nanotubules arrays as new materials for sensing and biosensing: Synthesis and characterization. Sens Actuators B Chem 111–112:526–531
Rao TN, Yagi I, Miwa T, Tryk DA, Fujishima A (1999) Electrochemical oxidation of NADH at highly boron-doped diamond electrodes. Anal Chem 71:2506–2511
Xing X, Shao M, Liu CC (1996) Electrochemical oxidation of dihydronicotinadmide adenine dinucleotide (NADH) on single crystal gold electrodes. J Electroanal Chem 406:83–90
Xing YC, Li L, Chusuei CC, Hull RV (2005) Sonochemical oxidation of multiwalled carbon nanotubes. Langmuir 21:4185–4190
Wang F, Hu S (2009) Electrochemical sensors based on metal and semiconductor nanoparticles. Microchim Acta 165:1–22
Guzmán C, Orozco G, Verde Y, Jiménez S, Godínez LA, Juaristi E et al (2009) Hydrogen peroxide sensor based on modified vitreous carbon with multiwall carbon nanotubes and composites of Pt nanoparticles–dopamine. Electrochim Acta 54:1728–1732
Ragupathy D, Gopalan AI, Lee KP (2010) Electrocatalytic oxidation and determination of ascorbic acid in the presence of dopamine at multiwalled carbon nanotube–silica network–gold nanoparticles based nanohybrid modified electrode. Sens Actuators B Chem 143:696–703
Lin J, He C, Zhang L, Zhang S (2009) Sensitive amperometric immunosensor for α-fetoprotein based on carbon nanotube/gold nanoparticle doped chitosan film. Anal Biochem 384:130–135
Valentini F, Palleschi G, Morales EL, Orlanducci S, Tamburri E, Terranova ML (2007) Functionalized single-walled carbon nanotubes modified microsensors for the selective response of epinephrine in presence of ascorbic acid. Electroanal 19:859–869
Lin J, He C, Zhao Y, Zhang S (2009) One-step synthesis of silver nanoparticles/carbon nanotubes/chitosan film and its application in glucose biosensor. Sens Actuators B Chem 137:768–773
Lim SH, Lin J, Li Q, You JK (2005) A glucose biosensor based on electrodeposition of palladium nanoparticles and glucose oxidase onto Nafion-solubilized carbon nanotube electrode. Biosens Bioelectron 20:2341–2346
Burke LD (1998) The electrochemistry of gold: II the electrocatalytic behaviour of the metal in aqueous media. Gold Bull 31:39–50
Laviron E (1974) Adsorption, autoinhibition and autocatalysis in polarography and in linear potential sweep voltammetry. J Electroanal Chem 52:355–393
Laviron E (1979) General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J Electroanal Chem 101:19–28
Pariente F, Tobalina F, Moreno G, Hernandez L, Lorenzo E, Abruna HD (1997) Mechanistic studies of the electrocatalytic oxidation of NADH and ascorbate at glassy carbon electrodes modified with electrodeposited films derived from 3, 4-dihydroxybenzaldehyde. Anal Chem 69:4065–4075
Chen J, Bao J, Cai C, Lu T (2004) Electrocatalytic oxidation of NADH at an ordered carbon nanotubes modified glassy carbon electrode. Anal Chim Acta 516:29–34
Deng C, Chen J, Chen XL, Xiao C, Nie Z, Yao S (2008) Boron-doped carbon nanotubes modified electrode for electroanalysis of NADH. Electrochem Commun 10:907–909
Zhang MG, Smith A, Gorski W (2004) Carbon nanotube–chitosan system for electrochemical sensing based on dehydrogenase enzymes. Anal Chem 76:5045–5050
Wu L, Zhang X, Ju H (2007) Detection of NADH and ethanol based on catalytic activity of soluble carbon nanofiber with low overpotential. Anal Chem 79:453–458
Prieto-Simón B, Fàbregas E (2004) Comparative study of electron mediators used in the electrochemical oxidation of NADH. Biosens Bioelectron 19:1131–1138
Behera S, Sampath S, Raj CR (2008) Electrochemical functionalization of a gold electrode with redox-active self-assembled monolayer for electroanalytical application. J Phys Chem C 112:3734–3740
Wang D, Li Y, Hasin P, Wu Y (2011) Preparation, characterization, and electrocatalytic performance of graphene—methylene blue thin films. Nano Res 4:124–130
Nassef HM, Radi A-E, O’Sullivan CK (2006) Electrocatalytic sensing of NADH on a glassy carbon electrode modified with electrografted o-aminophenol film. Electrochem Commun 8:1719–1725
Zhu L, Zhai J, Yang R, Tian C, Guo L (2007) Electrocatalytic oxidation of NADH with Meldola’s blue functionalized carbon nanotubes electrodes. Biosens Bioelectron 22:2768–2773
Zeng J, Wei W, Wu L, Liu X, Liu K, Li Y (2006) Fabrication of poly(toluidine blue O)/carbon nanotube composite nanowires and its stable low-potential detection of NADH. J Electroanal Chem 595:152–160
Radoi A, Compagnone D, Valcarcel MA, Placidi P, Materazzi S, Moscone D, Palleschi G (2008) Detection of NADH via electrocatalytic oxidation at single-walled carbon nanotubes modified with Variamine blue. Electrochimica Acta 53:2161–2169
Raj CR, Chakraborty S (2006) Carbon nanotubes–polymer–redox mediator hybrid thin film for electrocatalytic sensing. Biosens Bioelectron 22:700–706
Maleki N, Safavi A, Tajabadi F (2006) High-performance carbon composite electrode based on an ionic liquid as a binder. Anal Chem 78:3820–3826
Manso J, Mena ML, Yanez-Sedeno P, Pingarron JM (2008) Alcohol dehydrogenase amperometric biosensor based on a colloidal gold–carbon nanotubes composite electrode. Electrochimica Acta 53:4007–4012
Agui L, Pena-Farfal C, Yanez-Sedeno P, Pingarron JM (2007) Poly - (3 - methylthiophene)/carbon nanotubes hybrid composite-modified electrodes. Electrochimica Acta 52:7946–7952
Maroneze CM, Arenas LT, Luz RCS, Benvenutti EV, Landers R, Gushikem Y (2008) Meldola’s blue immobilized on a new SiO2/TiO2/graphite composite for electrocatalytic oxidation of NADH. Electrochimica Acta 53:4167–4175
Mai N, Liu X, Zeng X, Xing L, Wei W, Luo S (2010) Electrocatalytic oxidation of the reduced nicotinamide adenine dinucleotide at carbon ionic liquid electrode modified with polythionine/multi-walled carbon nanotubes composite. Microchim Acta 168:215–220
Cui L, Ai S, Shang K, Meng X, Wang C, Electrochemical determination of NADH using a glassy carbon electrode modified with Fe3O4 nanoparticles and poly-2,6-pyridinedicarboxylic acid, and its application to the determination of antioxidant capacity. Microchim Acta (in press) doi: 10.1007/s00604-011-0594-3
Gao Q, Sun M, Peng P, Qi H, Zhang C (2010) Electro-oxidative polymerization of phenothiazine dyes into a multilayer-containing carbon nanotube on a glassy carbon electrode for the sensitive and low-potential detection of NADH. Microchim Acta 168:299–307
Liu X, Li B, Wang X, Li C (2010) One-step construction of an electrode modified with electrodeposited Au/SiO2 nanoparticles, and its application to the determination of NADH and ethanol. Microchim Acta 171:399–405
Banks CE, Compton RG (2003) Exploring the electrocatalytic sites of carbon nanotubes for NADH detection: an edge plane pyrolytic graphite electrode study. Analyst 130:1232–1239
Rubianes MD, Rivas GA (2003) Carbon nanotubes paste electrode. Electrochem Commun 5:689–694
Tzouwara-Karayanni SM, Karayannis MI, Crouch SR (1993) Removal of ascorbic acid interference in the determination of glucose and sucrose in non-alcoholic beverages. Food Chem 48:95–98
Matsumoto K, Tsukatani T, Okajima Y (1995) Amperometric flow-injection determination of citric acid in food using free citrate lyase and coimmobilized oxalacetate decarboxylase and pyruvate oxidase. Electroanalysis 7:527–530
Acknowledgements
This work was financially supported by the Romanian Ministry of Education, Research and Innovation thorough PN-II-RU-TE-2009-1 national program, under the project identification code TE_44.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 270 kb)
Rights and permissions
About this article
Cite this article
Radoi, A., Litescu, SC., Eremia, S.A.V. et al. Electrochemical investigation of a glassy carbon electrode modified with carbon nanotubes decorated with (poly)crystalline gold. Microchim Acta 175, 97–104 (2011). https://doi.org/10.1007/s00604-011-0658-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-011-0658-4