Abstract
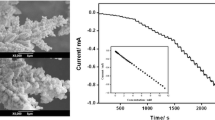
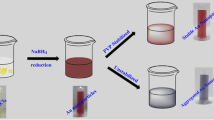
We describe a new method for the visualization of the activity of red-ox proteins on a gold interface. Glucose oxidase was selected as a model system. Surfaces were modified by adhesion of glucose oxidase on (a) electrochemically cleaned gold; (b) gold films modified with gold nanoparticles, (c) a gold surface modified with self-assembled monolayer, and (d) covalent immobilization of protein on the gold surface modified with a self-assembled monolayer. The simple optical method for the visualization of enzyme on the surfaces is based on the enzymatic formation of polypyrrole. The activity of the enzyme was quantified via enzymatic formation of polypyrrole, which was detected and investigated by quartz microbalance and amperometric techniques. The experimental data suggest that the enzymatic formation of the polymer may serve as a method to indicate the adhesion of active redox enzyme on such surfaces.

An optical method for the evaluation of activity and distribution of glucose oxidase on the different surfaces was described. The enzymatic synthesis of polypyrrole (black colour) was successfully applied for the visualization of active enzyme on the surfaces.





Similar content being viewed by others
References
Wang J (2008) Electrochemical glucose biosensors. Chem Rev 108:814
Ye JS, Wen Y, Zhang WD, Gan LM, Xu GQ, Sheu FS (2004) Nonenzymatic glucose detection using multi-walled carbon nanotube electrodes. Electrochem Commun 6:66
Zhu L, Li Y, Tian F, Xu B, Zhu G (2002) Electrochemiluminescent determination of glucose with a sol-gel derived ceramic-carbon composite electrode as a renewable optical fiber biosensor. Sens Actuat B: Chem 84:265
Shafer-Peltier KF, Haynes CL, Glucksberg MR, Van Duyne RR (2003) Toward a glucose biosensor based on surface-enhanced Raman scattering. J Am Chem Soc 125:588
Kong T, Yang C, Ye Y, Zhang K, Wang Z, Wang X (2009) An amperometric glucose biosensor based on the immobilization of glucose oxidase on the ZnO nanotubes. Sens Actuat B: Chem 138:344
Qui JD, Wang R, Liang RP, Xia XH (2009) Electrochemically deposited nanocomposite film of CS-Fc/AuNPs/GOx for glucose biosensor application. Biosens Bioelectron 24:2920
Cecchet F, Marcacio M, Margotti M, Paolucci F, Rapino S, Rudolf P (2006) Redox mediation at 11-mercaptoundecanoic acid self-assembled monolayers on gold. J Phys Chem B 110:2241
Ramanavicius A (2007) Amperometric biosensor for the determination of creatine. Anal Bioanal Chem 387:1899
Shervedani RK, Hatefi-Mehrjardi A (2009) Comparative electrochemical behavior of glucose oxidase covalently immobilized on mono-, di- and tetra-carboxylic acid functional Au-thiol SAMs via anhydride-derivatization route. Sens Actuat B 137:195
Gooding JJ, Erokhin P, Losic D, Yang W, Policarpio V, Liu J, Ho FM, Situmorang M, Hibbert DB, Shapter JG (2001) Parameters important in fabricating enzyme electrodes using self-assembled monolayers of alkanethiols. Anal Scien 17:3
Bourdillon C, Demaille C, Moiroux J, Savéant J-M (2006) From homogeneous electroenzymatic kinetics to antigen-antibody construction and characterization of spatially ordered catalytic enzyme assemblies on electrodes. Acc Chem Res 29:529
Hoshi T, Sagae N, Daikuhara K, Takahara K, Anzai J (2007) Multilayer membranes via layer-by-layer deposition of glucose oxidase and Au nanoparticles on a Pt electrode for glucose sensing. Mater Sci Eng C 27:890
Ramanavicius A, Malinauskas A, Ramanaviciene A (2004) Catalytic biosensors based on conducting polymers. In: Thomas DW (ed) Advanced biomaterials for medical applications. Kluwer Academic Publishers, Netherlands, p 93
Lehr J, Williamson BE, Barriere F, Downard AJ (2010) Dependence of catalytic activity and long-term stability of enzyme hydrogel films on curing time. Bioelectrochem 79:142
Ramanaviciene A, Schuhmann W, Ramanavicius A (2006) AFM study of conducting polymer polypyrrole nanoparticles formed by redox enzyme - glucose oxidase - initiated polymerisation. Coll Surf B 48:159
Kausaite-Minkstimiene A, Mazeiko V, Ramanaviciene A, Ramanavicius A (2010) Enzymatically synthesized polyaniline layer for extension of linear detection of amperometric glucose biosensor. Biosens Bioelectron 26:790
Losic D, Shapter JG, Gooding JJ (2002) Scanning tunneling microscopy studies of glucose oxidase on gold surfaces. Langmuir 18:5422
Zhu XL, Yang QL, Huang JY, Suzuki I, Li GX. Colorimetric study of the interaction between gold nanoparticles and a series of amino acids. J Nanosci Nanotechnol 8:353
Xiao Y, Patolsky F, Katz E, Hainfeld JF, Willner I (2003) “Plugging into enzymes”: nanowiring of redox enzymes by a gold nanoparticle. Science 299:1877
Thibault S, Aubriet H, Arnoult C, Ruch D (2008) Gold nanoparticles and a glucose oxidase based biosensor: an attempt to follow-up aging by XPS. Microchim Acta 163:211
Zhou N, Wang J, Chen T, Yu Z, Li G (2006) Enlargement of gold nanoparticles on the surface of a self-assembled monolayer modified electrode: a mode in biosensor design. Anal Chem 78:5227
Mrksich M, Sigal GB, Whitesides GM (1995) Surface-plasmon resonance permits in-situ measurement of protein adsorption on self-assembled monolayers of alkanethiolates on gold. Langmuir 11:4383
Pallarola D, Queralto N, Battaglini F, Azzaroni O (2010) Supramolecular assembly of glucose oxidase on concanavalin A-modified gold electrodes. Phys Chem Chem Phys 12:8071
Gooding JJ, Situmorang M, Erokhin P, Hibbert DB (1999) An assay for the determination of the amount of glucose oxidase immobilized in an enzyme electrode. Anal Commun 36:225
Im DM, Jang DH, Oh SM, Striebel Ch, Wiemhöfer HD, Gauglitz G, Göpel W (1995) Electrodeposited GOD/BSA electrodes: ellipsometric study and glucose-sensing behaviour. Sens Actuat B 24:149
Wang J, Carlisle JA (2006) Covalent immobilization of glucose oxidase on conducting ultrananocrystalline diamond thin films. Diam Rel Mat 15:279
Losic D, Shapter JG, Gooding JJ (2002) Scanning tunneling microscopy studies of glucose oxidase on gold surfaces. Langmuir 18:5422
Quinto M, Ciancio A, Zambonin PG (1998) A molecular resolution AFM study of gold-adsorbed glucose oxidase as influenced by enzyme concentration. J Electroanal Chem 448:51
Guiomar AJ, Guthrie JT, Evans SD (1999) Use of mixed self-assembled monolayers in a study of the effect of the microenvironment on immobilized glucose oxidase. Langmuir 15:1198
Raba J, Mottola HA (1995) Glucose oxidase as an analytical reagent. Crit Rev Anal Chem 25:1
Zhao J, Zhu HL, Lib T, Li GX (2008) Self-assembled multilayer of gold nanoparticles for amplified electrochemical detection of cytochrome c. Analyst 133:1242
Presnova G, Grigorenko V, Egorov A, Ruzgas T, Lindgren A, Gorton L, Börchers T (2000) Direct heterogeneous electron transfer of recombinant horseradish peroxidases on gold. Faraday Discus 116:281
Hecht HJ, Schomburg D, Kalisz H, Schmid RD (1993) The 3D structure of glucose oxidase from Aspergillus niger. Implications for the use of GOD as a biosensor enzyme. Biosens Bioelectron 8:197
De Benedetto GE, Malitesta C, Zambonin CG (1994) Electroanalytical/X-ray photoelectron spectroscopy investigation on glucose oxidase adsorbed on platinum. J Chem Soc, Faraday Trans 90:1495
Chi Q, Zhang J, Dong S, Wang E (1994) Direct observation of native and unfolded glucose oxidase structures by scanning tunnelling microscopy. J Chem Soc, Faraday Trans 90:2057
Szucs A, Hitchens GD, Bockris JO (1989) On the adsorption of glucose oxidase at a gold electrode. J Electrochem Soc 136:3748
Dong XD, Lu J, Cha C (1997) Characteristics of the glucose oxidase at different surfaces. Bioelectrochem Bioenerg 42:63
Liu S, Leech D, Ju H (2003) Application of colloidal gold in protein immobilization, electron transfer, and biosensing. Anal Lett 36:1
Stigter ECA, de Jong GJ, van Bennekom WP (2005) An improved coating for the isolation and quantitation of interferon-γ in spiked plasma using surface plasmon resonance (SPR). Biosens Bioelectron 21:474
Ramanavicius A, Kausaite A, Ramanaviciene A (2005) Polypyrrole coated glucose oxidase nanoparticles for biosensor design. Sens Actuat B: Chem 111–112:532
Ramanavicius A, Kausaite A, Ramanaviciene A, Acaite J, Malinauskas A (2006) Redox enzyme - glucose oxidase - initiated synthesis of polypyrrole. Synth Met 156:409
Ramanavicius A, Kausaite A, Ramanaviciene A (2008) Self-encapsulation of oxidases as a basic approach to tune upper detection limit of amperometric biosensors. Analyst 133:1083
Kausaite A, Ramanaviciene A, Ramanavicius A (2009) Polyaniline synthesis catalyzed by glucose oxidase. Polymer 50:1846
Acknowledgements
The authors would like to thank European Union Structural Funds project “Postdoctoral Fellowship Implementation in Lithuania”, SF-PD-2009-08-17-0151 for the financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
Below is the link to the electronic supplementary material.
ESM 1
(DOC 1348 kb)
Rights and permissions
About this article
Cite this article
Ramanaviciene, A., Kausaite-Minkstimiene, A., Oztekin, Y. et al. Visualization of red-ox proteins on the gold surface using enzymatic polypyrrole formation. Microchim Acta 175, 79–86 (2011). https://doi.org/10.1007/s00604-011-0645-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-011-0645-9