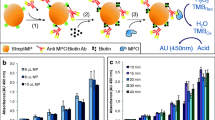
Abstract
A disposable electrochemical myeloperoxidase (MPO) immunosensor was fabricated based on the indium tin oxide electrode modified with a film composed of gold nanoparticles (AuNPs), poly(o-phenylenediamine), multi-walled carbon nanotubes and an ionic liquid. The composite film on the surface of the electrode was prepared by in situ electropolymerization using the ionic liquid as a supporting electrolyte. Negatively charged AuNPs were then adsorbed on the modified electrode via amine-gold affinity and to immobilize MPO antibody. Finally, bovine serum albumin was employed to block possible remaining active sites on the AuNPs. The modification of the electrode was studied by cyclic voltammetry and scanning electron microscopy. The factors affecting the performance of the immunosensor were investigated in detail using the hexacyanoferrate redox system. The sensor exhibited good response to MPO over two linear ranges (from 0.2 to 23.4 and from 23.4 to 300 ng.mL−1), with a detection limit of 0.05 ng.mL−1 (at an S/N of 3).

A disposable electrochemical immunosensor for myeloperoxidase based on the indium tin oxide electrode modified with an ionic liquid composite film composed of gold nanoparticles, poly(o-phenylenediamine) and carbon nanotubes.





Similar content being viewed by others
References
Apple FS, Voss E, Lund L, Preese L, Berger CR, Henry TD (1995) Cardiac troponin, CK-MB and myoglobin for the early detection of acute myocardial infarction and monitoring of reperfusion following thrombolytic therapy. Clin Chim Acta 237:59–66. doi:10.1016/0009-8981(95)06064-K
Eiserich JP, Baldus S, Brennan ML, Ma WX, Zhang CX, Tousson A, Castro L, Lusis AJ, Aldons J, William MN, White CR, Freeman BA (2002) Myeloperoxidase, a leukocyte-derived vascular NO oxidase. Science 29:2391–2394. doi:10.1126/science.1106830
Wang ZN, Nicholls SJ, Rodriguez ER, Kummu O, Horkko S, Barnard J, Reynolds WF, Topol EJ, DiDonato JA, Hazen SL (2007) Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat Med 10:1176–1184. doi:10.1038/nm1637
Fossard M, Fuchs I, Leitner JM, Hsieh K, Vlcek M, Losert H, Domanovits H, Schreiber W, Laggner AN, Jilma B (2004) Platelet function predicts myocardial damage in patients with acute myocardial infarction. Circulation 110:1392–1397. doi:10.1161/01.CIR.0000141575.92958.9C
Nicholls SJ, Hazen SL (2005) Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol 25:1102–1111. doi:10.1161/01.ATV.0000163262.83456.6d
Brennan ML, Penn MS, Lente FV, Nambi V, Shishehbor MH, Aviles RJ, Goormastic M, Pepoy ML, McErlean ES, Topol EJ, Nissen SE, Hazen SL (2003) Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med 17:1595–1604
Franck T, Grulke S, Dupont D, Deby C, Duvivier H, Peters F, Serteyn D (2005) Development of an enzyme-linked immunosorbent assay for specific equine neutrophil myeloperoxidase measurement in blood. J Vet Diagn Invest 17:412–419
Gandley RE, Rohland J, Zhou Y, Shibata E, Harger GF, Rajakumar A, Kagan VE, Markovic N, Hubel CA (2008) Increased myeloperoxidase in the placenta and circulation of women with preeclampsia. Hypertention 52:387–393. doi:10.1161/HYPERTENSIONAHA.107.107532
Liu K, Yuan R, Chai YQ, Tang DP, An HZ (2010) [AuCl4]− and Fe3+/[Fe(CN)6]3− ions-derivated immunosensing interface for electrochemical immunoassay of carcinoembryonic antigen in human serum. Bioproccess Biosyst Eng 33:179–185. doi:10.1007/s00449-009-0302-4
Wang XL, Tao GH, Meng YH (2009) Nanogold hollow microsphere-based electrochemical immunosensor for the detection of ferritin in human serum. Microchim Acta 167:147–152. doi:10.1007/s00604-009-0225-4
Kim DM, Noh HB, Park DS, Ryu SH, Koo JS, Shim YB (2009) Immunosensors for detection of Annexin II and MUC5AC for early diagnosis of lung cancer. Biosens Bioelectron 25:456–462. doi:10.1016/j.bios.2009.08.007
Mani V, Chikkaveeraiah BV, Patel V, Gutkind JS, Rusling JF (2009) Ultrasensitive immunosensor for cancer biomarker proteins using gold nanoparticle film electrodes and multienzyme-particle amplification. ACS Nano 3:585–594
Zhang SB, Zheng F, Wu ZS, Shen GL, Yu RQ (2008) Highly sensitive electrochemical etection of immunospecies based on combination of Fc label and PPD film/gold nanoparticle amplification. Biosens Bioelectron 24:129–135. doi:10.1016/j.bios.2008.03.017
Dai JH, Baker GL, Bruening ML (2006) Use of porous membranes modified with polyelectrolyte multilayers as substrates for protein arrays with low nonspecific adsorption. Anal Chem 78:135–140. doi:10.1021/ac0513966
Qian P, Ai SY, Yin HS, Li JH (2010) Evaluation of DNA damage and antioxidant capacity of sericin by a DNA electrochemical biosensor based on dendrimer-encapsulated Au-Pd/chitosan composite. Microchim Acta 168:347–354. doi:10.1007/s00604-009-0280-x
Cao Y, Wang J, Xu YY, Li GX (2010) Sensing purine nucleoside phosphorylase activity by using silver nanoparticles. Biosens Bioelectron 25:1032–1036. doi:10.1016/j.bios.2009.09.021
Huang KJ, Niu DJ, Xie WZ, Wang W (2010) A disposable electrochemical immunosensor for carcinoembryonic antigen based on nano-Au/multi-walled carbon nanotubes–chitosans nanocomposite film modified glassy carbon electrode. Anal Chim Acta 659:102–108. doi:10.1016/j.aca.2009.11.023
Deng CY, Li MR, Xie QJ, Liu ML, Tan YM, Xu XH, Yao SZ (2005) New glucose biosensor based on a poly(o-phenylendiamine)/glucose oxidase-glutaraldehyde/Prussian blue/Au electrode with QCM monitoring of various electrode-surface modifications. Anal Chim Acta 557:85–94. doi:10.1016/j.aca.2005.10.009
Zhang ZN, Liu HY, Deng JQ (1996) A glucose biosensor based on immobilization of glucose oxidase in electropolymerized o-Aminophenol film on platinized glassy carbon electrode. Anal Chem 68:1632–1638. doi:10.1021/ac950431d
Das J, Jo K, Lee JW, Yang HY (2007) Electrochemical immunosensor using p-Aminophenol redox cycling by hydrazine combined with a low background current. Anal Chem 79:2790–2796. doi:10.1021/ac0622911
Iijima S (1991) Helical microtubules of graphitic carbon. Nature 354:56–58
Gong K, Yan Y, Zhang M, Su L, Xiong S, Mao L (2005) Electrochemistry and electroanalytical application of carbon nanotubes: a review. Anal Sci 21:1383–1393
Huang B, Zhang WD, Chen CH, Yu YX (2010) Electrochemical determination of methyl parathion at a Pd/MWCNTs-modified electrode. Microchim Acta 171:57–62. doi:10.1007/s00604-010-0408-z
Zhang WD, Chen J, Jiang LC, Yu YX, Zhang JQ (2010) A highly sensitive nonenzymatic glucose sensor based on NiO-modified multi-walled carbon nanotubes. Microchim Acta 168:259–265. doi:10.1007/s00604-010-0288-2
Zhao HY, Xu XX, Zhang JX, Zheng W, Zheng YF (2010) Carbon nanotube-hydroxyapatite -hemoglobin nanocomposites with high bioelectrocatalytic activity. Bioelectrochemistry 78:124–129. doi:10.1016/j.bioelectrochem.2009.08.009
Zhang XH, Wang SM, Jia L, Xu ZX, Zeng Y (2008) An electrochemical sensor for determination of calcium dobesilate based on PoPD/MWNTs composite film modified glassy carbon electrode. J Biochem Biophys Methods 70:1203–1209. doi:10.1016/j.jbbm.2007.10.002
Dai Z, Xiao Y, Yu XZ, Mai ZB, Zhao XJ, Zou XY (2009) Direct electrochemistry of myoglobin based on ionic liquid-clay composite films. Biosens Bioelectron 24:1629–1634. doi:10.1016/j.bios.2008.08.032
Fukushima T, Kosaka A, Ishimura Y, Yamamoto T, Takigawa T, Ishii N, Aida T (2003) Molecular ordering of organic molten salts triggered by single-walled carbon nanotubes. Science 27:2072–2074
Wang Q, Yun YB, Zheng JB (2009) Nonenzymatic hydrogen peroxide sensor based on a polyaniline-single walled carbon nanotubes composite in a room temperature ionic liquid. Microchim Acta 167:153–157. doi:10.1007/s00604-009-0236-1
Zhang JJ, Wang JL, Zhu JJ, Xu JJ, Chen HY, Xu DK (2008) An electrochemical impedimetric arrayed immunosensor based on indium tin oxide electrodes and silver-enhanced gold nanoparticles. Microchim Acta 163:63–70. doi:10.1007/s00604-008-0944-y
Frens G (1973) Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat Phys 241:20–21
Du YF, Wang H, Zhang AJ, Lu JX (2007) Electrosynthesis of poly(o-phenylenediamine) in ionic liquid and its properties. Chin Sci Bull 52:2174–2178. doi:10.1007/s11434-007-0331-9
Wang Q, Tang H, Xie QJ, Jia XE, Zhang YY, Tan L, Yao SZ (2008) The preparation and characterization of poly(o-phenylenediamine)/gold nanoparticles interface for immunoassay by surface plasmon resonance and electrochemistry. Colloids Surf B 63:254–261. doi:10.1016/j.colsurfb.007.12.007
Tang DP, Yuan R, Chai YQ, Zhong X, Liu Y, Dai JY, Zhang LY (2004) Novel potentiometric immunosensor for hepatitis B surface antigen using a gold nanoparticle-based biomolecular immobilization method. Anal Biochem 333:345–350. doi:10.1016/j.ab.2004.06.035
Lu LS, Liu B, Liu CG, Xie GM (2010) Amperometric immunosensor for myeloperoxidase in human serum based on a multi-wall carbon nanotubes-ionic liquid-cerium dioxide film-modified electrode. Bull Korean Chem Soc 31:3259–3265. doi:10.5012/bkcs.2010.31.11.3259
Windmiller JR, Chinnapareddy S, Santhosh P, Halamek J, Chuang MC, Bocharova V, Tseng TF, Chou TY, Katz E, Wang J (2010) Strip-based amperometric detection of myeloperoxidase. Biosens Bioelectron 26:886–889. doi:10.1016/j.bios.2010.07.031
Lin KC, Kunduru V, Bothara M, Rege K, Prasad S, Ramakrishna BL (2010) Biogenic nanoporous silica-based sensor for enhanced electrochemical detection of cardiovascular biomarkers proteins. Biosens Bioelectron 25:2336–2342. doi:10.1016/j.bios.2010.03.032
Acknowledgments
This work was supported by the Nature Science Foundation of Chongqing city in China (CSTC2010BB5356).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 99 kb)
Rights and permissions
About this article
Cite this article
Liu, B., Lu, L., Li, Q. et al. Disposable electrochemical immunosensor for myeloperoxidase based on the indium tin oxide electrode modified with an ionic liquid composite film containing gold nanoparticles, poly(o-phenylenediamine) and carbon nanotubes. Microchim Acta 173, 513–520 (2011). https://doi.org/10.1007/s00604-011-0575-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-011-0575-6