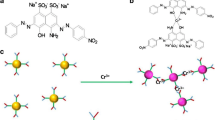
Abstract
We report on the first application of novel, water-soluble and fluorescent silver nanoclusters (Ag NCs) in a chemiluminescent (CL) detection system. A method has been developed for the determination of copper(II) ion that is based on the fact that the weak CL resulting from the redox reaction between Ce(IV) ion and sulfite ion is strongly enhanced by the Ag NCs and that the main CL signals now originate from Ag NCs. UV-visible spectra, CL spectra and fluorescent (FL) spectra were acquired to investigate the enhanced CL mechanism. It is proposed that the electronic energy of the excited state intermediate SO2* that originates from the CL reaction is transferred to Ag NCs to form an electronically excited NC whose emission is observed. In addition, it is found that copper(II) is capable of inhibiting the CL of the nanoclusters system, but not if other common metal ions are present. The detection of copper(II) is achieved indirectly by measuring the CL intensity of Ag NCs. Under the optimized experimental conditions, a linear relationship does exist between the intensity of CL and the concentrations of copper(II) in the range of 0.2 nM to 0.1 mΜ. The detection limit is 0.12 nM. The method is applied to the determination of copper(II) ion in tap water with satisfactory results.

We report the first application of novel, water-soluble and fluorescent silver nanoclusters in a chemiluminescent detection system. It was found that Ag NCs acted as the luminophor and energy acceptor. A method has been developed for the determination of copper(II) ion that is based on the fact that the capable of inhibiting the CL of the nanoclusters system.





Similar content being viewed by others
References
Toby S (1984) Chemiluminescence in the reactions of ozon. Chem Rev 84:277–285
Lin JM, Yamada M (2000) Chemiluminescent reaction of fluorescent organic compounds with KHSO5 using cobalt(II) as catalyst and its first application to molecular imprinting. Anal Chem 72:1148–1155
Juan AO, Francisco JB, Manuel C, Fernando DR (2004) Application of lanthanide-sensitised chemiluminescence to the determination of levofloxacin, moxifloxacin and trovafloxacin in tablets. Microchim Acta 144:207–213
Han HY, He ZK, Zeng YE (2006) Chemiluminescence method for the determination of glutathione in human serum using the Ru(phen) 2+3 –KMnO4 system. Microchim Acta 155:431–434
Godlewska-Żyłkiewicz B, Malejko J, Leśniewska B, Kojło A (2008) Assessment of immobilized yeast for the separation and determination of platinum in environmental samples by flow-injection chemiluminescence and electrothermal atomic absorption spectrometry. Microchim Acta 163:327–334
Li SF, Tao SJ, Wang FF, Hong JG, Wei XW (2010) Chemiluminescence reactions of luminol system catalyzed by nanoparticles of a gold/silver alloy. Microchim Acta 169:73–78
Wang X, Zhao HC, Nie LH, Jin LP, Zhang ZL (2001) Europium sensitized chemiluminescense determination of rufloxacin. Anal Chim Acta 445:169–175
Liu WB, Huang YM (2004) Cerium(IV)-based chemiluminescence of phentolamine sensitized by rhodamine 6 G. Anal Chim Acta 506:183–187
Yu J, Choi S, Dickson RM (2009) Shuttle-based fluorogenic silver-cluster biolabels. Angew Chem Int Ed 48:318–320
Vosch T, Antoku Y, Hsiang J, Richards CI, Gonzalez JI, Dickson RM (2007) Strongly emissive individual DNA-encapsulated Ag nanoclusters as single-molecule fluorophores. Proc Natl Acad Sci 104:12616–12621
Xie JP, Zheng YG, Ying JY (2009) Protein-directed synthesis of highly fluorescent gold nanoclusters. J Am Chem Soc 131:888–889
Patel SA, Richards CI, Hsiang JC, Dickson RM (2008) Water-soluble Ag nanoclusters exhibit strong two-photon-induced fluorescence. J Am Chem Soc 130:11602–11603
Yu J, Patel SA, Dickson RM (2007) In vitro and intracellular production of peptide-encapsulated fluorescent silver nanoclusters. Angew Chem Int Ed 46:2028–2030
Lin CAJ, Yang TY, Lee CH, Huang SH, Sperling RA, Zanella M, Li JK, Shen JL, Wang HH, Yeh HI, Parak WJ, Chang WH (2009) Synthesis, characterization, and bioconjugation of fluorescent gold nanoclusters toward biological labelin applications. Nano 3:3395–401
Triulzi RC, Micic M, Giordani S, Serry M, Chiou WA, Leblanc RM (2006) Immunoasssay based on the antibody-conjugated PAMAM-dendrimer-gold quantum dot complex. Chem Commun 48:5068–5070
Huang CC, Yang Z, Lee KH, Chang HT (2007) Synthesis of highly fluorescent gold nanoparticles for sensing mercury(II). Angew Chem Int Ed 46:6824–6828
Huang CC, Chiang CK, Lin ZH, Lee KH, Chang HT (2008) Bioconjugated gold nanodots and nanoparticles for protein assays based on photoluminescence quenching. Anal Chem 80:1497–1504
Zheng J, Dickson RM (2002) Individual water-soluble dendrimer-encapsulated silver nanodot fluorescence. J Am Chem Soc 124:13982–13983
Petty JT, Zheng J, Hud NV, Dickson RM (2004) DNA-templated Ag nanocluster formation. J Am Chem Soc 126:5207–5212
Ritchie CM, Johnsen KR, Kiser JR, Antoku Y, Dickson RM, Petty JT (2007) g Nanocluster formation using a cytosine oligonucleotide template. J Phys Chem C 111:175–181
Shen Z, Duan H, Frey H (2007) Water-soluble fluorescent Ag nanoclusters obtained from multiarm star poly(acrylic acid) as “Molecular Hydrogel” templates. Adv Mater 19:349–352
Shang L, Dong SJ (2008) Silver nanocluster-based fluorescent sensors for sensitive detection of Cu(II). J Mater Chem 18:1–6
Shang L, Dong SJ (2008) Facile preparation of water-soluble fluorescent silver nanoclusters using a polyelectrolyte template. Chem Commun 9:1088–1090
Cui H, Zhang ZF, Shi MJ, Xu Y, Wu YL (2005) Light emission of gold nanoparticles induced by the Reaction of bis(2, 4, 6-trichlorophenyl) oxalate and hydrogen peroxide. Anal Chem 77:6402–6406
Takeuchi K, Ibusuki T (1985) Determiomation of traces of hydrogensulfite by chemiluminescence with cerium(IV) sulfate as the reagent. Anal Chim Acta 174:359–363
Huang YM, Zhang C, Zhang XR, Zhang ZJ (1999) Chemiluminescence of sulfite based on auto-oxidation sensitized by rhodamine 6 G. Anal Chim Acta 391:95–100
Yu XJ, Jiang ZH, Wang QJ, Guo YS (2010) Silver nanoparticle-based chemiluminescence enhancement for the determination of norfloxacin. Microchim Acta 171:17–22
Zheng J, Nicovich PR, Dickson RM (2007) Highly fluorescent noble-metal quantum dots. Annu Rev Phys Chem 58:409–431
Sorouraddin MH, Manzoori JL, Iranifam M (2005) Determination of copper(II) based on its catalytic effect on thiosemicarbazide–H2O2–CTMAB chemiluminescence reaction. Talanta 66:1117–1121
Acknowledgements
This research was supported by the National Natural Science Foundation of China (No. 20505004), Outstanding Adult-young Scientific Research Encouraging Foundation of Shandong Province (No. 2008BS03015) and the Doctoral Found of QUST (No. 0022235).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Effect of Ag NCs concentration on the CL intensity, Ce(IV): 0.7 mM; Na2SO3: 0.6 mM; H2SO4: 45 mM (DOC 42 kb)
Fig. S2
Effect of Ce(IV) concentration on the CL intensity, Ag nanoclusters: 11 μM; Na2SO3: 0.6 mM; H2SO4: 45 mM. (DOC 37 kb)
Fig. S3
Effect of Na2SO3 concentration on the CL intensity, Ag nanoclusters:11 μM; Ce(IV): 0.5 mM; H2SO4: 45 mM. (DOC 41 kb)
Rights and permissions
About this article
Cite this article
Yu, X., Wang, Q. The determination of copper ions based on sensitized chemiluminescence of silver nanoclusters. Microchim Acta 173, 293–298 (2011). https://doi.org/10.1007/s00604-011-0549-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-011-0549-8