Abstract
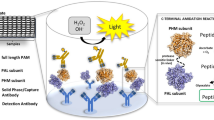
This study describes the development and validation of a highly sensitive and specific enzyme immunoassay (EIA) for therapeutic monitoring and pharmacokinetic studies of atorvastatin (ATR). The assay employs a polyclonal antibody that recognizes ATR with high specificity and affinity, and ATR conjugated to bovine serum albumin (ATR-BSA) immobilized onto microwell plates as a solid phase. The assay involved a competitive binding reaction between ATR and the immobilized ATR-BSA for the binding sites on a limiting amount of the anti-ATR antibody. The bound anti-ATR antibody was quantified with horseradish peroxidase-labeled anti-immunoglobulin secondary antibody and 3,3′,5,5′-tetramethylbenzidine as a substrate for the peroxidase enzyme. The concentration of ATR in the sample was quantified by its ability to inhibit the binding of the anti-ATR antibody to the immobilized ATR-BSA and subsequent color development in the assay wells. The conditions for the EIA were investigated and optimized for the determination of ATR in plasma samples. The limit of detection was 0.04 ng mL−1 and the effective working range at relative standard deviations (RSD) of ≤5% was 0.1–10 ng mL−1. Mean analytical recovery of ATR from spiked plasma was 99.3 ± 2.8%. The precision of the assay was satisfactory; RSD were 2.7–4.6 and 3.3–5.7% for intra- and inter-assay precision, respectively. The reliability of the EIA was confirmed by HPLC. The EIA is convenient, and one can analyze ∼ 200 samples per working day, facilitating the processing of large-number of samples of ATR.






Similar content being viewed by others
References
Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA (2002) Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. J Am Med Assoc 287:337–344
Block JH, Beale JM (2004) Organic medicinal and pharmaceutical chemistry, 11th edn. Lippincott Williams & Wilkins, New York
Bakker-Arkema RG, Davidson MH, Goldstein RJ, Davignon RJ, Isaacsohn SL, Weiss SR, Keilson LM, Brown WV, Miller VT, Shurzinske LJ (1996) Efficacy and safety of a new HMG-CoA reductase inhibitor, atorvastatin, in patients with hypertriglyceridemia. J Am Med Assoc 275:128–133
Shitara Y, Sugiyama Y (2006) Pharmacokinetic and pharmacodynamic alterations of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors: drug–drug interactions and interindividual differences in transporter and metabolic enzyme functions. Pharmacol Ther 112:71–105
Igel M, Sudhop T, Von-Bergmann K (2001) Metabolism and drug interactions of 3-hydroxy-3-methylglutaryl coenzyme A-reductase inhibitors (statins). Eur J Clin Pharmacol 57:357–364
Erturk S, Onal A, Cetin SM (2003) Analytical methods for the quantitative determination of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors in biological samples. J Chromatogr B 793:193–205
Pasha MK, Muzeeb S, Basha SJS, Shashikumar D, Mullangi R, Srinivas NR (2006) Analysis of five HMG-CoA reductase inhibitors: atorvastatin, lovastatin, pravastatin, rosuvastatin and simvastatin: pharmacological, pharmacokinetic and analytical overview and development of a new method for use in pharmaceutical formulations analysis and in vitro metabolism studies. Biomed Chromatogr 20:282–293
Nirogi R, Mudigonda K, Kandikere V (2007) Chromatography-mass spectrometry methods for the quantitation of statins in biological samples. J Pharm Biomed Anal 44:379–387
Novakova L, Satinsky D, Solich P (2008) HPLC methods for the determination of simvastatin and atorvastatin. TrAC 27:352–367
Zarghi A, Shafaati A, Foroutan SM, Khoddam A (2005) A simple and rapid HPLC method for the determination of atorvastatin in human plasma with UV detection and its application to pharmacokinetic studies. Arzneimittel Forschung 55:451–454
Bahrami G, Mohammadi B, Mirzaeei S, Kiani A (2005) Determination of atorvastatin in human serum by reversed-phase high-performance liquid chromatography with UV detection. J Chromatogr 826:41–45
Borek-Dohalsky V, Huclova J, Barrett B, Nemec B, Ulc I, Jelinek I (2006) Validated HPLC-MS-MS method for simultaneous determination of atorvastatin and 2-hydroxyatorvastatin in human plasma-pharmacokinetic study. Anal Bioanal Chem 386:275–285
Hermann M, Christensen H, Reubsaet JL (2005) Determination of atorvastatin and metabolites in human plasma with solid-phase extraction followed by LC-tandem MS. Anal Bioanal Chem 382:1242–1249
Darwish IA (2006) Immunoassay methods and their applications in pharmaceutical analysis: basic methodology and recent advances. Int J Biomed Sci 2:217–235
Gomez-Henz A, Aguilar-Caballos MP (2003) Stopped-flow fluorescence polarization immunoassay. Comb Chem High Throughput Screen 6:177–182
Suzuki Y, Arakawa H, Maeda M (2003) The immunoassay of methotrexate by capillary electrophoresis with laser-induced fluorescence detection. Anal Sci 19:111–115
Kobayashi N, Shibusawa K, Kubota K, Hasegawa N, Sun P, Niwa T, Junichi G (2003) Monoclonal anti-idiotype antibodies recognizing the variable region of a high-affinity antibody against 11-deoxycortisol: production, characterization and application to a sensitive noncompetitive immunoassay. J Immunol Methods 274:63–75
Ito K, Nakagawa K, Murakami S, Arakawa H, Maeda M (2003) Highly sensitive simultaneous bioluminescent measurement of acetate kinase and pyruvate phosphate dikinase activities using a firefly luciferase-luciferin reaction and its application to a tandem bioluminescent enzyme immunoassay. Anal Sci 19:105–109
Hermida J, Boveda MD, Vadillo FJ, Tutor JC (2002) Comparison between the Cobas Integra immunoassay and high-performance liquid chromatography for therapeutic monitoring of carbamazepine. Clin Biochem 35:251–254
Wen-Chi T, Pi-Ju RP (2009) Surface plasmon resonance-based immunosensor with oriented immobilized antibody fragments on a mixed self-assembled monolayer for the determination of staphylococcal enterotoxin B. Microchim Acta 166:115–122
Darwish IA, Al-Obaid AM, Al-Malaq HA (2009) Preparation of a highly specific polyclonal antibody against fluvastatin and its use in development of ELISA for determination of fluvastatin in plasma. Anal Methods 1:220–224
Habeeb AF (1966) Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal Biochem 14:328–336
Darwish IA, Al-Obaid AM, Al-Malaq HA (2009) Novel enzyme-linked immunosorbent assay for determination of fluvastatin in plasma at picogram level. Talanta 80:179–183
Anderson DJ (1989) Determination of the lower limit of detection. Clin Chem 35:2152–2153
Findlay JW, Smith WC, Lee JW, Nordblom GD, Das I, De Silva B, SKhan MN, Bowsher RR (2000) Validation of immunoassays for bioanalysis: a pharmaceutical industry perspective. J Pharm Biomed Anal 21:1249–1273
Hermida RC, Calvo C, Ayala DE, Lopez JE (2005) OR-6: administration time-dependent efficacy of valsartan-atorvastatin combination in hyperlipidemic patients with essential hypertension. Am J Hypertens 18:2A–2A
Eyal L, Bi M, Reuven Z, Angela F, Marina S, Dov G (2003) Treatment with amLodipine and atorvastatin have additive effect in improvement of arterial compliance in hypertensive hyperlipidemic patients. Am J Hypertens 16:715–718
Christie MB, John H, Alberto N, Lorenzo M, Leslie JL, Ramachandran S, Steven S, Alexandre PL, Philip TS, Enrico PV (2003) Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Circulation 107:2409–2415
Kiortsis DN, Milionis H, Rizos E, Elisaf MS (2000) Efficacy of combination of atorvastatin and micronized fenofibrate for the treatment of severe mixed lipid disorders. Atherosclerosis 151:133–134
Acknowledgment
The authors thank King Abdulaziz City for Science and Technology for funding the work (KACS-16-98AT).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Darwish, I.A., Al-Obaid, AR.M. & Al-Malaq, H.A. A highly sensitive and specific polyclonal antibody-based enzyme immunoassay for therapeutic monitoring and pharmacokinetic studies of atorvastatin. Microchim Acta 170, 67–74 (2010). https://doi.org/10.1007/s00604-010-0390-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-010-0390-5