Abstract
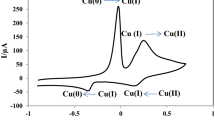
A novel route is presented for the fabrication of chromium (III) hexacyanoferrate(II) (Crhf) nanoparticles attached to single-walled carbon nanotubes (SWNTs) modified glassy carbon electrodes (GCE). The morphological character of the Crhf/SWNTs nanocomposites was examined using scanning electron microscopy (SEM), transmission electron microscopy, UV-Vis spectroscopy and Fourier transform infrared spectrometry. The performance of the Crhf/SWNTs nanocomposite modified electrode was characterized using cyclic voltammetry and amperometry. The electrode exhibits efficient electron transfer ability and high electrochemical response towards hydrazine. Moreover it showed a wide linear working range (0.5–500 μM hydrazine), with a detection limit of 0.45 μM estimated at a signal to noise ratio of 3. Response time is fast (about 3 s) and the electrode exhibited good reproducibility and long-term stability.





Similar content being viewed by others
References
Reddington E, Sapienza A, Gurau B, Viswanathan R, Sarangapani S, Smotkin ES et al (1998) Combinatorial electrochemistry: a highly parallel, optical screening method for the discovery of better electrocatalysts. Science 280:1735
Hogarth MP, Hards GA (1996) Direct methanol fuel cell. Platinum Met Rev 40:150
Ren XM, Wilson MS, Gottesfeld S (1996) High performance direct methanol polymer electrolyte fuel cells. J Electrochem Soc 143:12
Deivaraj TC, Chen WX, Lee JY (2003) Preparation of PtNi nanoparticles for the electrocatalytic oxidation of methanol. J Mater Chem 13:2555
Yang CC, Kumara AS, Kuo MC, Chien SH, Zena JM (2005) Copper–palladium alloy nanoparticle plated electrodes for the electrocatalytic determination of hydrazine. Anal Chim Acta 554:66
Nassef HM, Radi AE, O’Sullivan CK (2006) Electrocatalytic oxidation of hydrazine at o-aminophenol grafted modified glassy carbon electrode: Reusable hydrazine amperometric sensor. J Electroanal Chem 592:139
Revenga-Parra M, Lorenzo E, Pariente F (2005) Synthesis and electrocatalytic activity towards oxidation of hydrazine of a new family of hydroquinone salophen derivatives: application to the construction of hydrazine sensors. Sens Actuators B 107:678
Colonnese R, Ianniello RM (1989) Determination of Hydrazine in Polyvinylpyrrolidone by Derivatization and Square Wave Voltammetry. Microchim Acta 2:7
Neff VD (1978) Electrochemical oxidation and reduction of thin films of Prussian blue. J Electrochem Soc 125:886
Itaya K, Ataka T, Toshima S (1982) Electrochemical preparation of a Prussian blue analog: iron-ruthenium cyanide. J Am Chem Soc 104:3751
Ghosh PK, Bard AJ (1983) Clay-Modified Electrodes. J Am Chem Soc 105:5691
Murray CG, Nowak RJ, Rolison DR (1984) Electrogenerated coatings containing zeolites. J Electronanal Chem 164:205
Kulesza PJ, Faulkner LR (1988) Electrocatalysis at a novel electrode coating of nonstoichiometric tungsten (VI, V) oxide aggregates. J Am Chem Soc 110:4905
Zagal JH, Momoz E, Ureta-Zanartu S (1982) Catalytic electro-oxidation of hydrazine on phthalocyanines deposited on graphite electrodes. Electrochim Acta 27:1373
Collman JP, Deniserich P, Konai Y, Marrocco M, Koval C, Anson FC (1980) Electrode catalysis of the four-electron reduction of oxygen to water by dicobalt face-to-face porphyrins. J Am Chem Soc 102:6027
Opekar F (1989) Detection of hydrogen in air with a detector containing a nafion membrane metallized on both sides. J Electroanal Chem 260:451
Karyakin AA (2001) Prussian blue and its analogues electrochemistry and analytical applications. Electroanalysis 13:813
Kertesz V, Dunn NM, Berkel GJV (2002) Electrochemistry—electrospray-mass spectrometry study of cesium uptake in nickel hexacyanoferrate films. Electrochim Acta 47:1035
Eftekhari A (2003) Electrocatalysis and amperometric detection of hydrogen peroxide at an aluminum microelectrode modified with cobalt hexacyanoferrate fillm. Microchim Acta 141:15
Sheng QL, Shen Y, Zhang HF, Zheng JB (2008) Neodymium (III) hexacyanoferrate (II) nanoparticles induced by enzymatic reaction and their use in biosensing of glucose. Electrochimica Acta 53:4687
Zeng JX, Wei WZ, Liu XY, Wang Y, Luo GM (2008) A simple method to fabricate a Prussian blue nanoparticles/carbon nanotubes/poly(1,2-diaminobenzene) based glucose biosensor. Microchim Acta 160:261
Mu YY, Liang H, Hu J, Jiang L, Wan LJ (2005) Controllable Pt nanoparticle deposition on carbon nanotubes as an anode catalyst for direct methanol fuel cells. J Phys Chem B 109:22212
Xu CL, Chen JF, Cui Y, Han QY, Choo H, Liaw PK, Wu DH (2006) Influence of the surface treatment on the deposition of platinum nanoparticles on the carbon nanotubes. Adv Eng Mater 8:73
Zhao Y, Fan L, Zhong HZ, Li YF (2007) Electrodeposition and electrocatalytic properties of platinum nanoparticles on multi-walled carbon nanotubes: effect of the deposition conditions. Microchim Acta 158:327
Yan JY, Song HH, Yang SB, Yan JD, Chen XH (2008) Preparation and electrochemical properties of composites of carbon nanotubes loaded with Ag and TiO2 nanoparticle for use as anode material in lithium-ion batteries. Electrochimica Acta 53:6351
Ellis D, Eckhoff M, Neff VD (1981) Electrochromism in the mixed-Valence hexacyanides.1 Voltammetric and spectral studies of the oxidation and reduction of thin films of Prussian blue. J Phys Chem 85:1225
Wu P, Lu S, Cai CX (2004) Electrochemical preparation and characterization of a samarium hexacyanoferrate modified electrode. J Electroanal Chem 569:143
Zhang YJ, Wen Y, Liu Y, Li D, Li JH (2004) Functionalization of single-walled carbon nanotubeswith Prussian blue. Electrochem Commun 6:1180
Itaya K, Uchida I, Neff VD (1986) Electrochemistry of polynuclear transition metal cyanides: Prussian blue and its analogues. Acc Chem Res. 19:162
Lin MS, Tseng TF, Shih WC (1998) Chromium(III) hexacyanoferrate(II)-based chemical sensor for the cathodic determination of hydrogen peroxide. Analyst January 123:159
Brown DB, Shriver DF, Schwartz LH (1968) Solid-state reactions of iron (II) hexacyanochromate(III). Inorg Chem 7:77
Golabi SM, Zare HR (1999) Electrocatalytic oxidation of hydrazine at a chlorogenic acid (CGA) modified glassy carbon electrode. J Electroanal Chem 465:168
Zare HR, Nasirrizadeh N (2007) Hematoxylin multi-wall carbon nanotubes modified glassy carbon electrode for electrocatalytic oxidation of hydrazine. Electrochim Acta 52:4153
Umar A, Rahman MM, Kim SH, Hahn YB (2008) Zinc oxide nanonail based chemical sensor for hydrazine detection. Chem Commun 2:166
Acknowledgements
We appreciated the support of the National Natural Science Foundation of China (No.20675001), Science Foundation of Education Office of Anhui Province (No.2006kj145B) and Program for Innovative Research Team in Anhui Normal University.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supp Table 1
Literatures for electrocatalytic detection of hydrazine based on different modified electrode materials. (DOC 28.5 KB)
Supplementary Figure 1
SEM images of (A) pure SWNTs film and (B) Crhf/SWNTs nanocomposites. (DOC 173 KB)
Supplementary Figure 2
The corresponding histograms of size distribution of nanoparticles Crhf. (DOC 32.5 KB)
Supplementary Figure 3
CVs in 0.1 M PBS containinging 0.1 M KCl (pH 3.5), in the absence of hydrazine on (a) bare GCE and (b) Crhf/SWNTs/GCE. (DOC 227 KB)
Supplementary Figure 4
CVs of 5 × 10−5 M hydrazine at: (a) bare GCE; (b) SWNTs/GCE; (c) Crhf/SWNTs/GCE in 0.1 M PBS containing 0.1 M KCl at pH 3.5. Scan rate: 50 mV s−1. (DOC 310 KB)
Rights and permissions
About this article
Cite this article
Fang, B., Shen, R., Zhang, W. et al. Electrocatalytic oxidation of hydrazine at a chromium hexacyanoferrate/single-walled carbon nanotube modified glassy carbon electrode. Microchim Acta 165, 231–236 (2009). https://doi.org/10.1007/s00604-008-0125-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-008-0125-z