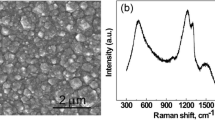
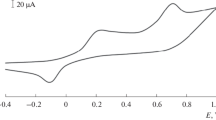
Abstract
A novel tyrosinase-based biosensor was developed for the determination of 2,4-dichlorophenol (2,4-DCP) by immobilizing tyrosinase on multi-walled carbon nanotubules (MWNTs) and polydiallyldimethylammonium chloride (PDDA) modified glassy carbon electrode. The biosensor showed a sensitive electrochemical response to 2,4-DCP in the presence of oxygen in solution. The effects of pH, adsorption time of PDDA, amount of tyrosinase immobilized on the enzyme electrode, and the volume of the MWNTs solution during the preparation of the sensor on the amperometric response of the electrode were explored for optimum analytical performance. The biosensor exhibited a fast amperometric response (less than 7 s), a high sensitivity and good storage stability for monitoring 2,4-DCP. The method showed good linearity in the range from 2 μm to 100 μm 2,4-DCP with a correlation coefficient of 0.997 and a detection limit of 0.66 μm. The response of the electrode showed Michaelis-Menten behavior at lower 2,4-DCP concentrations. The \(K_m^{{\text{app}}} \) value of immobilized tyrosinase on the modified electrode was calculated to be 66.3 μm using 2,4-DCP as the substrate.






Similar content being viewed by others
References
Ureta-Zañartu MS, Bustos P, Berrıíos C, Diez MC, Mora ML, Gutiérrez C (2002) Electrooxidation of 2,4-dichlorophenol and other polychlorinated phenols at a glassy carbon electrode. Electrochim Acta 47:2399
Liu B, Wang JP, Liu ML, Zhu HM (2004) Determination of 2,4-dichlophenol with internal standard method by gas chromatography. Spectrosc Lab 21:576
Wagner M, Nicell JA (2002) Detoxification of phenolic solutions with horseradish peroxidase and hydrogen peroxide. Wat Res 36:4041
Xu F, Bhandari A (2003) Retention and extractability of phenol, cresol and dichlorophenol exposed to two surface soils in the presence of horseradish peroxidase enzyme. Agric Food Chem 51:183
Yang S, Rudolf SSW, Kong RYC (2002) Biodegradation and enzymatic responses in the marine diatom Skeletonema costatum upon exposure to 2,4-dichlorophenol. Aquat Toxicol 59:191
Wang CC, Lee CM, Kuan CH (2000) Removal of 2,4-dichlorophenol by suspended and immobilized Bacillus insolitus. Chemosphere 41:447
Castillo M, Domingues R, Alpendurada MF, Barcelo D (1997) Persistence of selected pesticides and their phenolic transformation products in natural waters using off-line liquid solid extraction followed by liquid chromatographic techniques. Anal Chim Acta 353:133
Crespin MA, Gallego M, Valcarcel M (2002) Solid-phase extraction method for the determination of free and conjugated phenol compounds in human urine. J Chromatogr B Anal Technol Biomed Life Sci 773:89
Huang XJ, Qiu NN, Yuan DX (2008) Direct enrichment of phenols in lake and sea water by stir bar sorptive extraction based on poly (vinylpyridine–ethylene dimethacrylate) monolithic material and liquid chromatographic analysis. J Chromatogr A 1194:134
Takeda S, Tanaka Y, Yamane M, Siroma Z, Wakida S, Otsuka K, Terabe S (2001) Ionization of dichlorophenols for their analysis by capillary electrophoresis-mass spectrometry. J Chromatogr A 924:415
Ruedas Rama MJ, Ruiz Medina A, Molina Di’az A (2003) A simple and straight forward procedure for monitoring phenol compounds in waters by using UV solid phase transduction integrated in a continuous flow system. Microchim Acta 141:143
Li CY (2007) Voltammetric determination of 2-chlorophenol using a glassy carbon electrode coated with multi-wall carbon nanotube-dicetyl phosphate film. Microchim Acta 157:21
Huang SS, Qu YX, Li RN, Shen J, Zhu LW (2008) Biosensor based on horseradish peroxidase modified carbon nanotubes for determination of 2,4-dichlorophenol. Microchim Acta 162:261
Rajesh, Takashima W, Kaneto K (2004) Amperometric phenol biosensor based on covalent immobilization of tyrosinase onto an electrochemically prepared novel copolymer poly(N-3-aminopropyl pyrrole-co-pyrrole) film. Sens Actuators B Chem 102:271
Liu SQ, Yu JH, Ju HX (2003) Renewable phenol biosensor based on a tyrosinase–colloidal gold modified carbon paste electrode. J Electroanal Chem 540:61
Wang G, Xu JJ, Ye LH, Zhu JJ, Chen HY (2002) Highly sensitive sensors based on the immobilization of tyrosinase in chitosan. Bioelectrochem 57:33
Stephanie GB (1994) Biocatalysis with polyphenol oxidase: a review. Catal Today 22:459
Burestedt E, Narvaez A, Ruzgas T, Gorton L, Emnenus J, Dominguez E, Marko-Varga G (1996) Rate-limiting steps of tyrosinase-modified electrodes for the detection of catechol. Anal Chem 68:1605
Zhou Y, Li Z, Hu N, Zeng Y, Rusling JF (2002) Layer-by-layer assembly of ultrathin films of hemoglobin and clay nanoparticles with electrochemical and catalytic activity. Langmuir 18:8573
Lvov Y, Lovo Y, Mohwald H (2000) Protein architecture: Interfacing molecular assemblies and immobilization ,biotechnology. Marcel Dekker, New York, pp 125–167
Liu GD, Lin YH (2006) Amperometric glucose biosensor based on self-assembling glucose oxidase on carbon nanotubes. Electrochem Commun 8:251
Wang J, Abdel-Nasser K, Jan MR (2004) Carbon-nanotube-modified electrodes for amplified enzyme-based electrical detection of DNA hybridization. Biosens Bioelectron 20:995
Wei SH, Zhao FQ, Xu ZY, Zeng BZ (2006) Voltammetric determination of folic acid with a multi-walled carbon nanotube-modified gold electrode. Microchimica Acta 152:285
Yi HC, Qu WY, Huang WS (2008) Electrochemical determination of malachite green using a multi-wall carbon nanotube modified glassy carbon electrode. Microchimica Acta 160:291
Zhao H, Ju H (2006) Multilayer membranes for glucose biosensing via layer-by-layer assembly of multiwall carbon nanotubes and glucose oxidase. Anal Biochem 350:138
Zhao Q, Zhuang QK (2005) Determination of phenolic compounds based on the tyrosinase-single walled carbon nanotubes sensor. Electroanalysis 17(1):85
Zhang M, Yan Y, Gong K, Mao L, Guo Z, Chen Y (2004) Electrostatic layer-by-layer assembled carbon nanotube multilayer film and its electrocatalytic activity for O2 reduction. Langmuir 20:8781
Zhang MG, Smith A, Gorshi W (2004) Carbon nanotube–chitosan system for electrochemical sensing based on dehydrogenase enzymes. Anal Chem 76:5045
Qian L, Yang X (2006) Composite film of carbon nanotubes and chitosan for preparation of amperometric hydrogen peroxide biosensor amperometric hydrogen peroxide biosensor. Talanta 68:721
Cai CX, Chen J (2004) Direct electron transfer of redox proteins and enzymes promoted by carbon nanotube. Electrochemistry 10:159
Liu SN, Cai CX (2007) Immobilization and characterization of alcohol dehydrogenase on single-walled carbon nanotubes and its application in sensing ethanol. J Electroanal Chem 602:103
Kim GY, Moon SH (2008) Optimized coverage of gold nanoparticles at tyrosinase electrode for measurement of a pesticide in various water samples. J Hazard Mater 156:141
Kamin RA, Wilson GS (1980) Rotating ring-disk enzyme electrode for biocatalysis kinetic studies and characterization of the immobilized enzyme layer. Anal Chem 52(8):1198
Dempsey E, Diamond D, Collier A (2004) Development of a biosensor for endocrine disrupting compounds based on tyrosinase entrapped within a poly(thionine) film. Biosens Bioelectron 20:367
Rajesh, Takashima W, Kaneto K (2004) Amperometric tyrosinase based biosensor using an electropolymerized PTS-doped polypyrrole film as an entrapment support. React Funct Polym 59:163
Rodriguez I, Cela R (1997) Combination of solid-phase extraction procedures with gas chromatographic hyphenated techniques for chlorophenol determination in drinking water. Trac-Trends in Anal Chem 16:463
Acknowledgments
This work was supported by the Chinese National High-tech R&D Program (2007AA06Z402), Project of the Educational Administration Foundation of Shanghai Municipal Government (06ZZ17), and Shanghai Leading Academic Discipline Project (S30406).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kong, L., Huang, S., Yue, Z. et al. Sensitive mediator-free tyrosinase biosensor for the determination of 2,4-dichlorophenol. Microchim Acta 165, 203–209 (2009). https://doi.org/10.1007/s00604-008-0121-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-008-0121-3