Abstract
The world's first clinical cardiac xenotransplantation, using a genetically engineered pig heart with 10 gene modifications, prolonged the life of a 57-year-old man with no other life-saving options, by 60 days. It is foreseeable that xenotransplantation will be introduced in clinical practice in the United States. However, little clinical or regulatory progress has been made in the field of xenotransplantation in Japan in recent years. Japan seems to be heading toward a "device lag", and the over-importation of medical devices and technology in the medical field is becoming problematic. In this review, we discuss the concept of pig-heart xenotransplantation, including the pathobiological aspects related to immune rejection, coagulation dysregulation, and detrimental heart overgrowth, as well as genetic modification strategies in pigs to prevent or minimize these problems. Moreover, we summarize the necessity for and current status of xenotransplantation worldwide, and future prospects in Japan, with the aim of initiating xenotransplantation in Japan using genetically modified pigs without a global delay. It is imperative that this study prompts the initiation of preclinical xenotransplantation research using non-human primates and leads to clinical studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The world's first clinical cardiac xenotransplantation was performed on January 7, 2022, at the University of Maryland, using a genetically engineered pig heart with 10 gene modifications. A 57-year-old man with no other life-saving options survived for 60 days with this treatment [1]. Furthermore, experimental kidney and heart transplantation from genetically engineered pigs into human brain-dead cadavers at New York University showed that hyperacute rejection can be avoided [2]. Preclinical studies on organ transplantation using genetically modified pigs conducted in the U.S. in recent years have shown favorable results [3, 4]. Xenotransplantation will be introduced into clinical practice in the United States. However, although there has been steady development in basic research in the field of xenotransplantation in Japan, some of which is ahead of the world [5,6,7,8,9], there has been little clinical or regulatory progress. Japan seems to be moving toward a "device lag" [10] and the problem of over-importation of medical devices and technology [11] in the medical field still exists.
In this review, we summarize the necessity for and current status of xenotransplantation in the world, and the future prospects for xenotransplantation in Japan, which should be initiated, using genetically modified pigs, at the same time as the rest of the world.
Need for cardiac xenotransplantation in Japan
In recent years, remarkable progress has been made in the treatment of severe heart failure, including drug therapy with the "fantastic four" [12] and left ventricular assist device (LVAD) therapy using HeartMate 3 (Abbot, Abbott Park, IL, USA) [13]. However, heart transplantation remains the definitive treatment for end-stage heart disease [14] and a shortage of heart transplant donors in Japan is a major obstacle. Although the number of heart transplantations performed in Japan is increasing each year, only 79 were performed in 2022 [15], whereas the number of patients on the waiting list for heart transplant registered with the Japan Organ Transplantation Network was 867 in November, 2023 [16]. The average waiting time in Status 1 for the 79 heart transplant recipients in 2022 was 1769 days [15].
In Japan, where the waiting period for heart transplantation is long, LVADs are particularly important as a bridge to transplantation. Recently, the use of LVADs as the main treatment for patients who are not candidates for heart transplantation has become increasingly important. This LVAD treatment is called ‘a destination therapy’. In the United States, 78.1% of patients with LVAD implantations do not subsequently undergo heart transplantation [13]. The short-term outcomes of the latest magnetic levitation LVAD (HeartMate 3) are good, with reported 1- and 2-year patient survival rates of 85.9% and 78.8%, respectively [13]. According to data from the Japanese registry for Mechanically Assisted Circulatory Support, the 1- and 2-year patient survival rates for implantable LVADs in Japan are 93% and 90%, respectively [17]. However, the 10-year long-term outcomes of LVAD implantation remain unknown, and the complications associated with LVAD treatment cannot be ignored. Complications include bleeding and infection, which occur in 35% and 55% of patients, respectively [18]. Right heart failure is another important complication, occurring in approximately 30% of patients [19]. Right heart failure is a poor prognostic factor for LVAD, and complications of severe right heart failure decrease the chances of heart transplantation [20]. No implantable devices for right heart assistance are covered by health insurance, and patients with a biventricular assist device (BiVAD) using extracorporeal right heart support cannot be discharged from hospital. Although implantable BiVADs using two implantable devices for left heart assistance have been reported [21, 22], a retrospective study on 93 implantable BiVADs identified patient survival rates of 56% and 47% at 1- and 2 years, respectively, which were worse than those achieved by LVADs [23]. In another recent study, the median 1-year survival rate was 58.5%, with a median pump thrombosis rate of 31% (mainly right-sided) [24]. The outcomes of totally artificial hearts were worse than those of BiVADs [25].
Recently, cardiac tissue engineering has been used in drug discovery and human disease modeling, with the subsequent clinical applications of cardiac regeneration therapy using induced pluripotent stem cells [26, 27]. Moreover, 3D bioprinting has been used successfully to create cardiovascular structures, substituting blood vessels, heart valves, and myocardium [28]. However, the creation of a perfect substitute heart has not been achieved, and its realization is likely to take decades.
For these reasons, cardiac xenotransplantation, defined as the use of animal hearts for transplantation, is expected to become a viable alternative to the allotransplantation of hearts from humans. Between January, 2010 and July, 2022, 133 patients required temporary mechanical circulatory support such as venoarterial extracorporeal membrane oxygenation (V-A ECMO), temporary extracorporeal ventricular assist devices, and Impella (Abiomed, Danvers, MA, USA) at Osaka University Hospital (Fig. 1). The temporary device was removed following functional recovery in 61 of these patients, and 37 patients were approved as heart transplantation or destination therapy-LVAD (DT-LVAD) candidates by the institutional committee. The remaining 35 patients were not candidates for human heart allotransplantation or DT-LVAD and could not be weaned off mechanical support. The clinical outcomes of these patients were poor, with a median survival of only 3.2 months (0.8–35.8 months). Such patients are potential candidates for cardiac xenotransplantation.
Between January, 2010 and July, 2022, a total of 133 patients required temporary mechanical circulatory support at Osaka University Hospital. Of these 133 patients, 35 were not candidates for human allotransplantation or left ventricular assist devices as destination therapy and could not be weaned off mechanical support. The clinical outcomes of these 35 patients were poor; however, such patients are possible candidates for cardiac xenotransplantation. HTx heart transplantation with human hearts, MCS mechanical circulatory support
History of clinical cardiac xenotransplantation
The world's first clinical xenotransplantation was conducted before Barnard performed the first human heart allotransplantation in 1967 [29]. In 1964, Hardy transplanted a chimpanzee heart into a human (Table 1). However, the transplanted heart was unable to assist the recipient's circulation sufficiently [30]. Subsequent attempts at transplantation using non-human primates (NHPs), sheep and pig hearts also failed [31,32,33,34]. Then, in 1984, Baily transplanted a baboon heart into a female neonate (Baby Fae), who survived for 20 days [35]. This greatly stimulated the subsequent development of pediatric cardiac allotransplantation. Subsequently, Religa [36] and Baruah [37] performed heart transplants from pigs in 1992 and 1996, respectively, and documented survival of 23 h and 7 days, respectively. Clinical cardiac xenotransplantation was not attempted thereafter for a long time [31].
On January 7, 2022, cardiac xenotransplantation of the heart of a genetically engineered pig was performed at the University of Maryland [1]. This case is discussed in detail in the following section. The second procedure was performed on September 20, 2023 [38].
Usefulness of pigs as xenotransplant donors
NHPs, which are more closely related to humans immunologically, are advantageous as xenograft donors. In previous clinical xenotransplants, only those using baboon hearts avoided hyperacute rejection [35]. However, beyond the fact that the organs of NHPs are difficult to obtain, their use in human transplantation is not ethically acceptable.
Although pigs are immunologically discordant to humans, they are considered useful and viable xenotransplant donors for the following reasons [39]:
-
Available in size and function equivalent to a human heart.
-
High reproductive performance (high fecundity), short developmental time to sexual maturity and adult size allow for efficient multiplication.
-
Ethical acceptance for the use of organs to save human lives.
-
The natural lifespan of a pig is approximately 15–20 years, and a comparable lifespan of an organ can be expected.
-
Pigs can be bred under designated pathogen-free (DPF) conditions to reduce the risk of disease transmission.
-
Efficient and accurate gene modification techniques have been established to overcome immunological problems.
Immunological issues in transplanting pig organs into humans
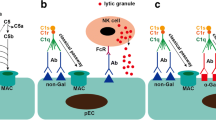
All humans and NHPs possess natural antibodies against the surface antigens of pig cells. When porcine organs are transplanted into humans or NHPs, these natural antibodies immediately bind to the vascular endothelial cells of the graft. Some activate the complement pathway (antibody-mediated complement activation), whereas others attract leukocytes that adhere to and infiltrate through Fc-receptor-mediated and Fc-independent mechanisms. This reaction usually occurs within minutes to hours [40] and is called hyperacute rejection (HAR). The histopathological features of hyperacute rejection include venous thrombosis, loss of vascular integrity, interstitial hemorrhage, edema, and innate immune cell infiltration [41,42,43].
The natural antigens responsible for HAR have been found to be against carbohydrate antigens on the surface of porcine cells. Mammals other than humans, apes, and old world monkeys have a glycosyltransferase called alpha1,3-galactosyltransferase (alpha1,3GT or GGTA1) [44], and this enzyme causes the expression of galactose-α(1.3)-galactose (αGal) on the cell surface. Humans, apes, and old-world monkeys have lost the GGTA1 gene; therefore, they do not express αGal on the cell surface. Primates are believed to acquire natural antibodies against αGal when microorganisms colonize their gastrointestinal tracts during infancy because these microorganisms have the same surface antigens on their cells [45]. More recently compared with the loss of the GGTA1 gene (approximately 3.5 million years ago), humans have lost the function of another enzyme involved in sialic acid synthesis: cytidine monophosphate-N-acetyl-neuramic acid hydroxylase (CMAH) [46], resulting in the absence of N-glycolylneuraminic acid (Neu5Gc). However, Neu5Gc is expressed on glycoproteins and glycolipids in most organs and cells of mammals, including pigs, and the diet-based intake of Neu5Gc provokes natural immunization and the production of anti-Neu5Gc antibodies in human serum. [47, 48]. Moreover, NHPs have natural antibodies against the sugar chain corresponding to the human Sd(a) blood group antigen synthesized by the β-1,4-N-acetyl-galactosaminyl transferase 2 (β4GalNT2) gene [49, 50]. Conversely, most humans have the Sd(a) antigen, and it is unclear whether the Sd(a) antigen produced by pig β4GalNT2 shows antigenicity in most humans [51].
Although the most important mechanism in hyperacute rejection is antibody-mediated complement activation, it is known that the complement itself can recognize and kill xenogeneic cells thorough an alternative pathway [52]. Cells of the innate immune system such as natural killer (NK) cells, macrophages, and neutrophils also play a more important role in xenotransplantation than in allotransplantation [9].
Modification of porcine cell surface antigenicity by gene modification technology
The first genetic alterations in pigs focused on human complement regulatory proteins (CRPs), such as membrane cofactor protein (MCP: CD46), decay-accelerating factor (DAF: CD55), and CD59 [8]. First, DAF transgenic pigs were produced in 1994, followed by transgenic pigs expressing other CRPs [53].
On the contrary, even after αGal was found to be the main cause of HAR, the knockout of αGal was not easy. This is because unlike in mice, porcine embryonic stem cells have not yet been established. Therefore, competitive inhibition and remodeling of Gal epitopes by the overexpression of α1,2fucosyltransferase, α2,3sialyltransferase [54], β-D-mannoside β-1,4-N-acetylglucosaminyltransferase III (GnT-III) [5] and others were investigated. In 2002, the technology of "Dolly," a cloned sheep, was applied to develop an αGal knockout pig by combining gene engineering and nuclear transplantation technologies into fetal fibroblasts [55] (Fig. 2). This technology was used to eliminate the major carbohydrate antigens on porcine cells, αGal, Neu5Gc, and Sd(a) epitopes, which are the targets of human natural antibodies. Three genes, GGTA1 [56], CMAH [57, 58], and β4GalNT2 [59], were removed and so-called triple-knockout (TKO) pigs were created. Significantly lower binding of natural antibodies to TKO pig-derived cells than to wild-type pig cells in human blood has been confirmed [60].
Somatic cell cloning is a technique in which the nuclei of cultured somatic cells are implanted into the cytoplasm of unfertilized eggs to create cloned embryos, and cloned individuals are born through a borrowed abdominal pregnancy. By applying procedures such as gene transfer or gene knockout to cultured somatic cells, genetically modified cell nuclei can be obtained. Using such nuclei to create cloned embryos leading to cloned individuals, a theoretically unlimited number of genetically modified individuals can be produced as required
Once the anti-pig antibody targets are eliminated, antibody-dependent cellular cytotoxicity (ADCC) by NK cells is attenuated. However, there is direct human NK cell cytotoxicity against pig cells because human NK cell receptors cannot bind to swine leukocyte antigen (SLA)-I. To address this issue, it is possible to suppress human NK cell activity by expressing human leukocyte antigen (HLA)-E on pig cell surfaces [61, 62]. Human macrophages are also activated by porcine cells. This is because CD47 on pig cells cannot bind to the “don't eat me” signal regulatory protein alpha (SIRPα) on human macrophages. Therefore, TG pigs expressing human CD47 were generated, and these pig cells were reported to be protected from human monocyte- or macrophage-mediated cellular cytotoxicity [63, 64]. Several other molecules that regulate monocytes/macrophages have been identified over the past decade. For example, HLA-G1 [65] and HLA-E [66] were found to inhibit monocytes/macrophages and NK cells. Monocytes and macrophages share many receptors in common with NK cells. Alterations in carbohydrate antigens, such as the overexpression of α2,6 sialic acid, are also effective in controlling monocyte/macrophage [67, 68]. Moreover, it has been reported that CD31 and CD177 can be used to control neutrophils [9].
Methods to control acquired immunity
In pig-to-human heart transplantation, even if hyperacute rejection by natural antibodies, acute humoral xenograft rejection, and innate cellular xenograft rejection by innate immune cells can be prevented, adaptive cellular xenograft rejection, in which T- and B cells play major roles, is expected. The activation of human/NHP T cells in pig organs occurs either directly by antigen-presenting cells (APCs) of the pig or indirectly by APCs of human/NHPs. Several co-stimulatory and co-inhibitory signals are involved in this process [39]. The direct activation of T cells can be attenuated by the removal or downregulation of SLA molecules, which are the major histocompatibility complexes in pigs [69, 70]. Moreover, the blockade of co-stimulatory signals of T cell activation has been found to be effective. Among them, the CD40-CD154 co-stimulation blockade is considered important in pig-to-human/NHP transplantation. Since 2000, anti-CD154 monoclonal antibodies have been used in xenotransplantation experiments [71]; however, since anti-CD154 monoclonal antibodies are thrombogenic in humans, anti-CD40 monoclonal antibodies-based regimens have been established and shown good results for pig-to-baboon heterotopic [72] and orthotopic heart transplantation [3, 73].
Measures against disorders of the coagulation system
Another facet of the pathobiology of pig organ xenotransplantation is dysregulation of the coagulation pathway [74, 75]. The contributing mechanisms include the human immune response to porcine organs, which triggers inflammation, vascular injury, and the procoagulant surface of pig endothelial cells. The molecular incompatibility of coagulation regulators among pigs, humans, and NHPs is also important. Thrombotic microangiopathy (TM) can occur and cause organ damage, despite immunological adjustments to avoid hyperacute xenograft rejection and systemic anticoagulation therapy [76, 77]. The complex of thrombomodulin (TBM) and thrombin encounters protein C flowing in the blood and converts this protein into activated protein C. Activated protein C breaks down activated factor VIII (VIIIa) and activated factor V (Va) produced in the coagulation reaction, and fibrin is no longer formed, thus halting fibrin clot formation. Pig TBM is unable to bind to human thrombin and activate human protein C; therefore, coagulation inhibition does not occur, whereas TM does. The expression of human TBM in donor pig cells can circumvent TM [78]. Furthermore, the function of human TBM is enhanced by the expression of the human endothelial protein C receptor [79].
Other genetic modifications
In addition to the above genetic modifications of the immune and coagulation systems, the expression of anti-inflammatory proteins, such as human TNF-alpha-induced protein 3 (TNFAIP3, also known as A20) [80] and human heme oxygenase 1 (HMOX1) [81], have been found to be effective in protecting xenografts. The harmful overgrowth of pig hearts has been observed consistently in pig-NHP xenotransplantation in preclinical studies [73]. One solution to this problem is to create donor pigs with a loss-of-function mutation in the growth hormone receptor (GHR) gene [82, 83]. In orthotopic cardiac xenotransplantation between pigs and NHPs, GHR knockout improved outcomes, with 9 months of recipient-animal survival documented [3, 84].
Xenozoonosis control
Genetically modified donor pigs can be maintained in DPF facilities to achieve a high microbiological and virological safety profile [85, 86]. Furthermore, highly sensitive and specific testing methods have been established for specific pathogens that should not be present in donor pigs [87]. In Japan, Otabi et al. developed a panel consisting of 76 highly sensitive polymerase chain reaction (PCR) detection assays that could screen 41 viruses, 1 protozoan, and a broad range of bacteria [88]. Among pig-specific pathogens, porcine cytomegalovirus (pCMV) has been reported to compromise graft survival [89]. In the University of Maryland case, pCMV activation was mentioned as a possible cause of transplanted organ failure [90]. Moreover, porcine endogenous retroviruses (PERVs) are integrated into the porcine genome. However, PERV infection has not been detected in numerous preclinical xenotransplantation studies in NHPs or other species, in in vivo infection experiments in different species [91], or in clinical xenotransplantation of porcine islet cells in patients with diabetes [92, 93]. Attempts to knock out all PERVs from the porcine genome using clustered regularly interspaced short palindromic repeats/CRISPR-associated proteins (CRISPR/Cas9) technology have already been successful [94, 95].
Review of the case at the Maryland University
The patient was a 57-year-old man with mild chronic thrombocytopenia, hypertension, non-ischemic cardiomyopathy, and a history of mitral valve repair [1, 90]. He was hospitalized for severe heart failure with a left ventricular ejection fraction of 10% and required extensive intravenous inotropic support, which was escalated to intra-aortic balloon pumping and V-A ECMO. His case was reviewed by two regional and two prominent national heart transplantation programs, but the request for a transplant or LVAD was denied by all four programs because of poor adherence to treatment. The Food and Drug Administration approved cardiac xenotransplantation as an expanded access; that is, a potential pathway for a patient with a serious or immediately life-threatening disease or condition to gain access to an investigational medical product (drug, biological, or medical device) for treatment outside clinical trials.
Table 2 summarizes the 10 gene edits applied to the donor pigs. The source animal was derived from a PERV–C–negative line and tested every 3 months for pathogens that affect porcine or human health, including PERV-A, PERV-B, PERV-C, pCMV, and porcine lymphotropic herpesvirus. Table 3 lists the immunosuppressive drugs used in this patient.
During the transplantation procedure, the patient suffered aortic dissection from the aortic cross clamp and underwent repair. The residual dissection caused an occlusion of an upper-pole left renal artery and an endovascular stent was placed postoperatively. However, acute renal failure persisted, and renal replacement therapy was required. The chest was closed on day 2 after the transplantation and the patient was extubated. ECMO was discontinued on day 4, and the Swan-Ganz catheter was removed on day 6 with stable hemodynamics. On day 12, exploratory laparotomy was required but no signs of acute ischemia or perforation was observed. Because of the complicated postoperative course, the first endomyocardial biopsy was not performed until postoperative day 34, which revealed no evidence of rejection. The patient was rehabilitated without any cardiovascular support, and the xenograft functioned normally without evidence of rejection. On day 43, hypotension developed, and the patient was reintubated. Quantitative PCR was performed on peripheral blood mononuclear cells, which tested positive for pCMV. The patient had hypogammaglobulinemia; therefore, intravenous immunoglobulin was administered, and the antiviral therapy was changed from ganciclovir to cidofovir. The patient was extubated on day 47. On day 49, the patient’s hemodynamics suddenly deteriorated, and VA-ECMO was restarted. Echocardiography showed a dramatic increase in left and right ventricular wall thicknesses. Repeated endomyocardial biopsy was negative for pathologic antibody-mediated rejection on day 50, but positive on day 56. The xenograft dysfunction did not improve, and life support was withdrawn on day 60 after transplantation.
After thorough investigation, the transplantation team concluded that there were three possible causes of graft dysfunction: endogenous xenoantibody-mediated rejection, the exogenous administration of intravenous immunoglobulin -containing xenoantibodies, and reactivation of pCMV within the xenograft [90].
Current situation and prospects in Japan
Japan has led the world in basic research on xenotransplantation. When the mechanism of rejection in xenotransplantation was still unknown, Miyagawa et al. conducted a study using a guinea pig-to-rat heterotopic heart transplantation and concluded that the alternative pathway of complement activation is involved in the hyperacute rejection that occurs in this discordant combination. He reported that this response was related to the species specificity between complement and CRPs, which triggered a surge of research in the field of xenotransplantation worldwide (8). Since then, world-leading results have been reported from Japan, especially in the fields of complement, innate immunity, and glycan research (5–9).
Conversely, there has been no remarkable progress in clinical studies and regulation. In Japan, the ‘Regenerative medicine promotion act’ was passed and promulgated in 2013 and introduced the same year. The “Act on the safety of regenerative medicine” and the “Revised pharmaceutical affairs act” were passed and promulgated in 2013, and introduced in 2014 [96]. The ‘Act on the safety of regenerative medicine’ covers three classes of risk-dependent procedures: high-risk (Class I), medium-risk (Class II), and low-risk (Class III). Class I covers procedures involving human embryonic stem cells, induced pluripotent stem (iPS) cells, cells like iPS cells, cells in which a gene is introduced, and xenogeneic and allogeneic cells. Thus, the “Act on the safety of regenerative medicine” and the “Revised pharmaceutical affairs act” cover xenogeneic cell transplantation, including islet xenotransplantation, but not xenogeneic organ transplantation. The regulation of xenogeneic organ transplantation is currently under discussion. In the near future, xenogeneic organs, such as the heart and kidneys are expected to be treated as “regenerative medical products.”
The Research and Development Division, Health Policy Bureau, the Ministry of Health, Labour and Welfare (MHLW), updated the “Public Health Guidelines on Infectious Disease Issues in Xenotransplantation” in 2016, from the original 2001 publication [96]. The objective of this study was to prevent infections and the spread of emerging infectious diseases caused by xenotransplantation, making it relevant to public health.
Clinical trials are ready to begin in some areas of the world, whereas Japan seems to be behind other countries. Regarding regulation, there has been no progress since the “Public Health Guidelines on Infectious Disease Issues in Xenotransplantation” was revised in 2016 and the “Act on the Safety of Regenerative Medicine” was introduced in 2014. However, substantial research funds from the Japan Agency for Medical Research and Development have been allocated for islet xenotransplantation [97]. In February, 2022, an informal meeting was held between the MHLW and the Japanese Society for xenotransplantation to provide information on clinical trials in the United States and discuss the need for laws and regulations in xenotransplantation in Japan. Subsequently, an MHLW study group was formed and discussions on the formulation of regulations specifically for xenotransplantation were initiated [98]. The Japanese Government is currently focusing on clinical xenotransplantation. We hope that the Japanese government will provide large grants for research on xenotransplantation of the heart, kidneys, and other organs soon. It is imperative that this study prompts the initiation of preclinical xenotransplantation research using NHPs and leads to clinical studies.
References
Griffith BP, Goerlich CE, Singh AK, Rothblatt M, Lau CL, Shah A, et al. Genetically modified porcine-to-human cardiac xenotransplantation. N Engl J Med. 2022;387:35–44.
Montgomery RA, Stern JM, Lonze BE, Tatapudi VS, Mangiola M, Wu M, et al. Results of two cases of pig-to-human kidney xenotransplantation. N Engl J Med. 2022;386:1889–98.
Mohiuddin MM, Goerlich CE, Singh AK, Zhang T, Tatarov I, Lewis B, et al. Progressive genetic modifications of porcine cardiac xenografts extend survival to 9 months. Xenotransplantation. 2022;29: e12744.
Adams AB, Lovasik BP, Faber DA, Burlak C, Breeden C, Estrada JL, et al. Anti-C5 antibody tesidolumab reduces early antibody-mediated rejection and prolongs survival in renal xenotransplantation. Ann Surg. 2021;274:473–80.
Miyagawa S, Murakami H, Takahagi Y, Nakai R, Yamada M, Murase A, et al. Remodeling of the major pig xenoantigen by N-acetylglucosaminyltransferase III in transgenic pig. J Biol Chem. 2001;276:39310–9.
Fujita T, Miyagawa S, Ezoe K, Saito T, Sato N, Takahagi Y, et al. Skin graft of double transgenic pigs of N-acetylglucosaminyltransferase III (GnT-III) and DAF (CD55) genes survived in cynomolgus monkey for 31 days. Transpl Immunol. 2004;13:259–64.
Komoda H, Miyagawa S, Omori T, Takahagi Y, Murakami H, Shigehisa T, et al. Survival of adult islet grafts from transgenic pigs with N-acetylglucosaminyltransferase-III (GnT-III) in cynomolgus monkeys. Xenotransplantation. 2005;12:209–16.
Miyagawa S, Maeda A, Toyama C, Kogata S, Okamatsu C, Yamamoto R, et al. Aspects of the complement system in new era of xenotransplantation. Front Immunol. 2022;13: 860165.
Maeda A, Kogata S, Toyama C, Lo PC, Okamatsu C, Yamamoto R, et al. The innate cellular immune response in xenotransplantation. Front Immunology. 2022;13: 858604.
Murakami M, Suzuki Y, Tominaga T. Rapid globalization of medical device clinical development programs in Japan—the case of drug-eluting stents. Circ J. 2018;82:636–43.
The Ministry of Health, Labour and Welfare. Current status, problems and demands of the medical device industry (in Japanese). https://www.mhlw.go.jp/stf/shingi/2r9852000001y7lc-att/2r9852000001y7xb.pdf. Accessed 5 Jan 2024.
Bauersachs J. Heart failure drug treatment: the fantastic four. Eur Heart J. 2021;42:681–3.
Jorde UP, Saeed O, Koehl D, Morris AA, Wood KL, Meyer DM, et al. The society of thoracic surgeons interagency registry for mechanically sssisted circulatory support 2023 annual report: focus on magnetically levitated devices. Ann Thorac Surg. 2023;117:33–44.
Hsich E, Singh TP, Cherikh WS, Harhay MO, Hayes D Jr, Perch M, et al. The international thoracic organ transplant registry of the international society for heart and lung transplantation: Thirty-ninth adult heart transplantation report-2022; focus on transplant for restrictive heart disease. J Heart Lung Transplant. 2022;41:1366–75.
Japan Organt Transplantation Network. Heart Transplant Registry of Japan. http://www.jsht.jp/registry/japan/. Accessed 5 Jan 2024.
Japan Organt Transplantation Network. Number of registered transplant applicants. https://www.jotnw.or.jp/data/. Accessed 5 Jan 2024.
Council of Academic Societies Related to Mechanically Assisted Circulatory Support. J-MACS statistical report. https://j-vad.jp/document/statistical_report_20230215.pdf. Accessed 5 Jan 2024.
McNamara N, Narroway H, Williams M, Brookes J, Farag J, Cistulli D, et al. Contemporary outcomes of continuous-flow left ventricular assist devices-a systematic review. Ann Cardiothorac Surgery. 2021;10(2):186–208.
Mehra MR, Uriel N, Naka Y, Cleveland JC Jr, Yuzefpolskaya M, Salerno CT, et al. A fully magnetically levitated left ventricular assist device-Final report. N Engl J Med. 2019;380:1618–27.
Saito S, Toda K, Nakamura T, Miyagawa S, Yoshikawa Y, Hata H, et al. Rescuing patients with severe biventricular failure in the era of continuous-flow left ventricular assist device. Circ J. 2019;83:379–85.
Saito S, Sakaguchi T, Miyagawa S, Yoshikawa Y, Yamauchi T, Ueno T, et al. Biventricular support using implantable continuous-flow ventricular assist devices. J Heart Lung Transplant. 2011;30:475–8.
Saito S, Sakaguchi T, Sawa Y. Clinical report of long-term support with dual Jarvik 2000 biventricular assist device. J Heart Lung Transplant. 2011;30:845–7.
Marasco S, Simon AR, Tsui S, Schramm R, Eifert S, Hagl CM, et al. International experience using a durable, centrifugal-flow ventricular assist device for biventricular support. J Heart Lung Transplant. 2020;39:1372–9.
Farag J, Woldendorp K, McNamara N, Bannon PG, Marasco SF, Loforte A, et al. Contemporary outcomes of continuous-flow biventricular assist devices. Ann Cardiothorac Surg. 2021;10:311–28.
Arabía FA, Cantor RS, Koehl DA, Kasirajan V, Gregoric I, Moriguchi JD, et al. Interagency registry for mechanically assisted circulatory support report on the total artificial heart. J Heart Lung Transplant. 2018;37:1304–12.
Miyagawa S, Sawa Y. Building a new strategy for treating heart failure using Induced Pluripotent Stem Cells. J Cardiol. 2018;72:445–8.
Miyagawa S, Kainuma S, Kawamura T, Suzuki K, Ito Y, Iseoka H, et al. Case report: Transplantation of human induced pluripotent stem cell-derived cardiomyocyte patches for ischemic cardiomyopathy. Front Cardiovasc Med. 2022;9: 950829.
Liu N, Ye X, Yao B, Zhao M, Wu P, Liu G, et al. Advances in 3D bioprinting technology for cardiac tissue engineering and regeneration. Bioact Mater. 2021;6:1388–401.
Barnard CN. The operation. A human cardiac transplant: an interim report of a successful operation performed at Groote Schuur Hospital, Cape Town. S Afr Med J. 1967;41:1271–4.
Hardy JD, Kurrus FD, Chavez CM, Neely WA, Eraslan S, Turner MD, et al. Heart transplantation in man. Developmental studies and report of a case. JAMA. 1964;188:1132–40.
Taniguchi S, Cooper DK. Clinical xenotransplantation: past, present and future. Ann R Col Surg Engl. 1997;79:13–9.
Cooley DA, Hallman GL, Bloodwell RD, Nora JJ, Leachman RD. Human heart transplantation. Experience with twelve cases. Am J Cardiol. 1968;22:804–10.
Dureau, Fradin, Gonin, Michaud, Mikaeloff. Heart and liver transplantations (in French). Lyon Med. 1969;222:585–6.
Barnard CN, Wolpowitz A, Losman JG. Heterotopic cardiac transplantation with a xenograft for assistance of the left heart in cardiogenic shock after cardiopulmonary bypass. S Afr Med J. 1977;52:1035–8.
Bailey LL, Nehlsen-Cannarella SL, Concepcion W, Jolley WB. Baboon-to-human cardiac xenotransplantation in a neonate. JAMA. 1985;254:3321–9.
Czaplicki J, Blońska B, Religa Z. The lack of hyperacute xenogeneic heart transplant rejection in a human. J Heart Lung Transplant. 1992;11:393–7.
Jayaraman KS. Pig heart transplant surgeon held in jail. Nature. 1997;385:378.
Kounang N. Groundbreaking transplant of pig heart into living recipient is performed for the second time ever. CNN health. https://edition.cnn.com/2023/09/22/health/pig-heart-transplant-living-patient-second-time-ever/index.html. Accessed 5 Jan 2024
Reichart B, Langin M, Denner J, Schwinzer R, Cowan PJ, Wolf E. Pathways to clinical cardiac xenotransplantation. Transplantation. 2021;105:1930–43.
Lexer G, Cooper DK, Rose AG, Wicomb WN, Rees J, Keraan M, et al. Hyperacute rejection in a discordant (pig to baboon) cardiac xenograft model. J Heart Transplant. 1986;5:411–8.
Rose AG, Cooper DK, Human PA, Reichenspurner H, Reichart B. Histopathology of hyperacute rejection of the heart: experimental and clinical observations in allografts and xenografts. J Heart Lung Transplant. 1991;10:223–34.
Rose AG, Cooper DK. A histopathologic grading system of hyperacute (humoral, antibody-mediated) cardiac xenograft and allograft rejection. J Heart Lung Transplant. 1996;15:804–17.
Rose AG, Cooper DK. Venular thrombosis is the key event in the pathogenesis of antibody-mediated cardiac rejection. Xenotransplantation. 2000;7:31–41.
Galili U, Shohet SB, Kobrin E, Stults CL, Macher BA. Man, apes, and Old World monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J Biol Chem. 1988;263:17755–62.
Galili U, Mandrell RE, Hamadeh RM, Shohet SB, Griffiss JM. Interaction between human natural anti-alpha-galactosyl immunoglobulin G and bacteria of the human flora. Infect Immun. 1988;56:1730–7.
Chou HH, Takematsu H, Diaz S, Iber J, Nickerson E, Wright KL, et al. A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc Natl Acad Sci USA. 1998;95(20):11751–6.
Salama A, Evanno G, Harb J, Soulillou JP. Potential deleterious role of anti-Neu5Gc antibodies in xenotransplantation. Xenotransplantation. 2015;22:85–94.
Miwa Y, Kobayashi T, Nagasaka T, Liu D, Yu M, Yokoyama I, et al. Are N-glycolylneuraminic acid (Hanganutziu-Deicher) antigens important in pig-to-human xenotransplantation? Xenotransplantation. 2004;11:247–53.
Byrne G, Ahmad-Villiers S, Du Z, McGregor C. B4GALNT2 and xenotransplantation: a newly appreciated xenogeneic antigen. Xenotransplantation. 2018;25: e12394.
Sykes M, Sachs DH. Transplanting organs from pigs to humans. Sci Immunol. 2019;4:eaau6298.
Lo PC, Eguchi H, Sakai R, Maeda A, Kogata S, Toyama C, et al. Reactions to porcine cells with or without β4GalNT2. Transplant Proc. 2020;52:1916–8.
Komoda H, Miyagawa S, Kubo T, Kitano E, Kitamura H, Omori T, et al. A study of the xenoantigenicity of adult pig islets cells. Xenotransplantation. 2004;11:237–46.
Fukuta D, Miyagawa S, Kubo T, Matsunami K, Shirasu A, Hattori H, et al. Effect of hybrid complement regulatory proteins on xenogeneic cells. Biochem Biophys Res Commun. 2003;306:476–82.
Tanemura M, Miyagawa S, Koyota S, Koma M, Matsuda H, Tsuji S, et al. Reduction of the major swine xenoantigen, the alpha-galactosyl epitope by transfection of the alpha2,3-sialyltransferase gene. J Biol Chem. 1998;273:16421–5.
Dai Y, Vaught TD, Boone J, Chen SH, Phelps CJ, Ball S, et al. Targeted disruption of the alpha1,3-galactosyltransferase gene in cloned pigs. Nat Biotechnol. 2002;20:251–5.
Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003;299:411–4.
Kwon DN, Lee K, Kang MJ, Choi YJ, Park C, Whyte JJ, et al. Production of biallelic CMP-Neu5Ac hydroxylase knock-out pigs. Sci Rep. 2013;3:1981.
Lutz AJ, Li P, Estrada JL, Sidner RA, Chihara RK, Downey SM, et al. Double knockout pigs deficient in N-glycolylneuraminic acid and galactose α-1,3-galactose reduce the humoral barrier to xenotransplantation. Xenotransplantation. 2013;20:27–35.
Estrada JL, Martens G, Li P, Adams A, Newell KA, Ford ML, et al. Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/β4GalNT2 genes. Xenotransplantation. 2015;22:194–202.
Li Q, Hara H, Banks CA, Yamamoto T, Ayares D, Mauchley DC, et al. Anti-pig antibody in infants: can a genetically engineered pig heart bridge to allotransplantation? Ann Thorac Surg. 2020;109:1268–73.
Matsunami K, Kusama T, Okura E, Shirakura R, Fukuzawa M, Miyagawa S. Involvement of position-147 for HLA-E expression. Biochem Biophys Res Commun. 2006;347:692–7.
Weiss EH, Lilienfeld BG, Müller S, Müller E, Herbach N, Kessler B, et al. HLA-E/human beta2-microglobulin transgenic pigs: protection against xenogeneic human anti-pig natural killer cell cytotoxicity. Transplantation. 2009;87:35–43.
Ide K, Wang H, Tahara H, Liu J, Wang X, Asahara T, et al. Role for CD47-SIRPalpha signaling in xenograft rejection by macrophages. Proc Natl Acad Sci U S A. 2007;104:5062–6.
Maeda A, Lo PC, Sakai R, Noguchi Y, Kodama T, Yoneyama T, et al. A strategy for suppressing macrophage-mediated rejection in xenotransplantation. Transplantation. 2020;104:675–81.
Esquivel EL, Maeda A, Eguchi H, Asada M, Sugiyama M, Manabe C, et al. Suppression of human macrophage-mediated cytotoxicity by transgenic swine endothelial cell expression of HLA-G. Transpl Immunol. 2015;32:109–15.
Maeda A, Kawamura T, Ueno T, Usui N, Eguchi H, Miyagawa S. The suppression of inflammatory macrophage-mediated cytotoxicity and proinflammatory cytokine production by transgenic expression of HLA-E. Transpl Immunol. 2013;29:76–81.
Sakai R, Maeda A, Choi TV, Lo PC, Jiaravuthisan P, Shabri AM, et al. Human CD200 suppresses macrophage-mediated xenogeneic cytotoxicity and phagocytosis. Surg Today. 2018;48:119–26.
Noguchi Y, Maeda A, Lo PC, Takakura C, Haneda T, Kodama T, et al. Human TIGIT on porcine aortic endothelial cells suppresses xenogeneic macrophage-mediated cytotoxicity. Immunobiology. 2019;224:605–13.
Ladowski JM, Hara H, Cooper DKC. The role of SLAs in xenotransplantation. Transplantation. 2021;105:300–7.
Hammer SE, Ho CS, Ando A, Rogel-Gaillard C, Charles M, Tector M, et al. Importance of the major histocompatibility complex (swine leukocyte antigen) in swine health and biomedical research. Annu Rev Anim Biosci. 2020;8:171–98.
Samy KP, Butler JR, Li P, Cooper DKC, Ekser B. The role of costimulation blockade in solid organ and islet xenotransplantation. J Immunol Res. 2017;2017:8415205.
Mohiuddin MM, Singh AK, Corcoran PC, Thomas Iii ML, Clark T, Lewis BG, et al. Chimeric 2C10R4 anti-CD40 antibody therapy is critical for long-term survival of GTKO.hCD46.hTBM pig-to-primate cardiac xenograft. Nat Commun. 2016;7:11138.
Längin M, Mayr T, Reichart B, Michel S, Buchholz S, Guethoff S, et al. Consistent success in life-supporting porcine cardiac xenotransplantation. Nature. 2018;564:430–3.
Cowan PJ, Robson SC. Progress towards overcoming coagulopathy and hemostatic dysfunction associated with xenotransplantation. Int J Surg. 2015;23:296–300.
Bühler L, Basker M, Alwayn IP, Goepfert C, Kitamura H, Kawai T, et al. Coagulation and thrombotic disorders associated with pig organ and hematopoietic cell transplantation in nonhuman primates. Transplantation. 2000;70:1323–31.
Shimizu A, Hisashi Y, Kuwaki K, Tseng YL, Dor FJ, Houser SL, et al. Thrombotic microangiopathy associated with humoral rejection of cardiac xenografts from alpha1,3-galactosyltransferase gene-knockout pigs in baboons. Am J Pathol. 2008;172:1471–81.
Mohiuddin MM, Corcoran PC, Singh AK, Azimzadeh A, Hoyt RF Jr, Thomas ML, et al. B-cell depletion extends the survival of GTKO.hCD46Tgpig heart xenografts in baboons for up to 8 months. Am J Transplant. 2012;12:763–71.
Iwase H, Ekser B, Satyananda V, Bhama J, Hara H, Ezzelarab M, et al. Pig-to-baboon heterotopic heart transplantation–exploratory preliminary experience with pigs transgenic for human thrombomodulin and comparison of three costimulation blockade-based regimens. Xenotransplantation. 2015;22:211–20.
Salvaris EJ, Moran CJ, Roussel JC, Fisicaro N, Robson SC, Cowan PJ. Pig endothelial protein C receptor is functionally compatible with the human protein C pathway. Xenotransplantation. 2020;27: e12557.
Oropeza M, Petersen B, Carnwath JW, Lucas-Hahn A, Lemme E, Hassel P, et al. Transgenic expression of the human A20 gene in cloned pigs provides protection against apoptotic and inflammatory stimuli. Xenotransplantation. 2009;16:522–34.
Petersen B, Ramackers W, Lucas-Hahn A, Lemme E, Hassel P, Queisser AL, et al. Transgenic expression of human heme oxygenase-1 in pigs confers resistance against xenograft rejection during ex vivo perfusion of porcine kidneys. Xenotransplantation. 2011;18:355–68.
Iwase H, Ball S, Adams K, Eyestone W, Walters A, Cooper DKC. Growth hormone receptor knockout: relevance to xenotransplantation. Xenotransplantation. 2021;28: e12652.
Hinrichs A, Riedel EO, Klymiuk N, Blutke A, Kemter E, Längin M, et al. Growth hormone receptor knockout to reduce the size of donor pigs for preclinical xenotransplantation studies. Xenotransplantation. 2021;28: e12664.
Goerlich CE, Griffith B, Hanna P, Hong SN, Ayares D, Singh AK, et al. The growth of xenotransplanted hearts can be reduced with growth hormone receptor knockout pig donors. J Thorac Cardiovasc Surg. 2023;165:e69–81.
Fishman JA. Infectious disease risks in xenotransplantation. Am J Transplant. 2018;18:1857–64.
Fishman JA. Prevention of infection in xenotransplantation: Designated pathogen-free swine in the safety equation. Xenotransplantation. 2020;27: e12595.
Denner J. Sensitive detection systems for infectious agents in xenotransplantation. Xenotransplantation. 2020;e12594. https://doi.org/10.1111/xen.12594.
Otabi H, Miura H, Uryu H, Kobayashi-Harada R, Abe K, Nakano K, et al. Development of a panel for detection of pathogens in xenotransplantation donor pigs. Xenotransplantation. 2023;30(6):e12825. https://doi.org/10.1111/xen.12825.
Denner J, Längin M, Reichart B, Krüger L, Fiebig U, Mokelke M, et al. Impact of porcine cytomegalovirus on long-term orthotopic cardiac xenotransplant survival. Sci Rep. 2020;10:17531.
Mohiuddin MM, Singh AK, Scobie L, Goerlich CE, Grazioli A, Saharia K, et al. Graft dysfunction in compassionate use of genetically engineered pig-to-human cardiac xenotransplantation: a case report. Lancet. 2023;402:397–410.
Denner J. Why was PERV not transmitted during preclinical and clinical xenotransplantation trials and after inoculation of animals? Retrovirology. 2018;15:28.
Wynyard S, Nathu D, Garkavenko O, Denner J, Elliott R. Microbiological safety of the first clinical pig islet xenotransplantation trial in New Zealand. Xenotransplantation. 2014;21:309–23.
Morozov VA, Wynyard S, Matsumoto S, Abalovich A, Denner J, Elliott R. No PERV transmission during a clinical trial of pig islet cell transplantation. Virus Res. 2017;227:34–40.
Yang L, Güell M, Niu D, George H, Lesha E, Grishin D, et al. Genome-wide inactivation of porcine endogenous retroviruses (PERVs). Science. 2015;350:1101–4.
Niu D, Wei HJ, Lin L, George H, Wang T, Lee IH, et al. Inactivation of porcine endogenous retrovirus in pigs using CRISPR-Cas9. Science. 2017;357:1303–7.
Shimoda M, Matsumoto S. Update regarding xenotransplantation in Japan. Xenotransplantation. 2019;26: e12491.
Kobayashi T, Miyagawa S. Current activity of xenotransplantation in Japan. Xenotransplantation. 2019;26: e12487.
Kobayashi T. Status of Preparation for Clinical Application of Xenotransplantation (in Japanese). Jinkouzouki (Jpn J Artif Organs). 2023;52:215–20.
Acknowledgements
This research was supported by Japan Agency for Medical Research and Development (AMED) under Grant Number JP23mk0121265.
Funding
Open Access funding provided by Osaka University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saito, S., Miyagawa, S., Kawamura, T. et al. How should cardiac xenotransplantation be initiated in Japan?. Surg Today (2024). https://doi.org/10.1007/s00595-024-02861-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00595-024-02861-7