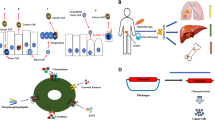
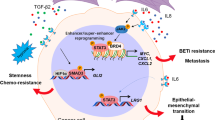
Abstract
The “bystander effect” is a transmission phenomenon mediating communication from target to non-target cells, as well as cell-to-cell interactions between neighboring and distantly located cells. In this narrative review, we describe the fundamental and clinical significance of the bystander effect with respect to cell-to-cell interactions in carcinogenesis, therapeutic response, and tissue regeneration. In carcinogenesis, the bystander effect mediates communications between tumor microenvironments and non-malignant epithelial cells and has been suggested to impact heterogeneous tumorigenic cells in tumors and cancerized fields. In therapeutic response, the bystander effect mediates communications between drug-sensitive and drug-resistant cells and may transmit both drug efficacy and resistance. Therefore, control of therapeutic response transmission via the bystander effect might offer a promising future cancer treatment. Finally, in tissue regeneration, circulating cells and stromal cells may differentiate into various cells for the purpose of tissue regeneration under direction of the bystander effect arising from surrounding cells in a defective space. We hope that the findings we present will promote the development of innovative cancer therapies and tissue regeneration methodologies from the viewpoint of cell-to-cell interactions through the bystander effect.




Similar content being viewed by others
References
Mothersill C, Rusin A, Fernandez-Palomo C, Seymour C. History of bystander effects research 1905-present; what is in a name? Int J Radiat Biol. 2018;94:696–707.
Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. Nanotubular highways for intercellular organelle transport. Science. 2004;303:1007–10.
Sherer NM, Mothes W. Cytonemes and tunneling nanotubules in cell-cell communication and viral pathogenesis. Trends Cell Biol. 2008;18:414–20.
Oshima A. Structure and closure of connexin gap junction channels. FEBS Lett. 2014;588:1230–7.
Majka M, Janowska-Wieczorek A, Ratajczak J, Ehrenman K, Pietrzkowski Z, Kowalska MA, et al. Numerous growth factors, cytokines, and chemokines are secreted by human CD34(+) cells, myeloblasts, erythroblasts, and megakaryoblasts and regulate normal hematopoiesis in an autocrine/paracrine manner. Blood. 2001;97:3075–85.
Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963–8.
Prevo LJ, Sanchez CA, Galipeau PC, Reid BJ. p53-mutant clones and field effects in Barrett’s esophagus. Cancer Res. 1999;59:4784–7.
Damania D, Roy HK, Subramanian H, Weinberg DS, Rex DK, Goldberg MJ, et al. Nanocytology of rectal colonocytes to assess risk of colon cancer based on field cancerization. Cancer Res. 2012;72:2720–7.
Curtius K, Wright NA, Graham TA. An evolutionary perspective on field cancerization. Nat Rev Cancer. 2018;18:19–32.
Baba Y, Ishimoto T, Kurashige J, Iwatsuki M, Sakamoto Y, Yoshida N, et al. Epigenetic field cancerization in gastrointestinal cancers. Cancer Lett. 2016;375:360–6.
Dotto GP. Multifocal epithelial tumors and field cancerization: stroma as a primary determinant. J Clin Invest. 2014;124:1446–53.
Choi CR, Bakir IA, Hart AL, Graham TA. Clonal evolution of colorectal cancer in IBD. Nat Rev Gastroenterol Hepatol. 2017;14:218–29.
Reid BJ, Li X, Galipeau PC, Vaughan TL. Barrett’s oesophagus and oesophageal adenocarcinoma: time for a new synthesis. Nat Rev Cancer. 2010;10:87–101.
Hazelton WD, Curtius K, Inadomi JM, Vaughan TL, Meza R, Rubenstein JH, et al. The role of gastroesophageal reflux and other factors during progression to esophageal adenocarcinoma. Cancer Epidemiol Biomarkers Prev. 2015;24:1012–23.
Morita M, Kuwano H, Ohno S, Seo Y, Tomoda H, Furusawa M, et al. Multiple occurrence of carcinoma in the upper aerodigestive tract associated with esophageal cancer: reference to smoking, drinking and family history. Int J Cancer. 1994;58:207–10.
Morita M, Saeki H, Mori M, Kuwano H, Sugimachi K. Risk factors for esophageal cancer and the multiple occurrence of carcinoma in the upper aerodigestive tract. Surgery. 2002;131:S1-6.
Miyazaki T, Sohda M, Higuchi T, Tanaka N, Suzuki S, Sakai M, et al. Effectiveness of FDG-PET in screening of synchronous cancer of other organs in patients with esophageal cancer. Anticancer Res. 2014;34:283–7.
Kuwano H, Ohno S, Matsuda H, Mori M, Sugimachi K. Serial histologic evaluation of multiple primary squamous cell carcinomas of the esophagus. Cancer. 1988;61:1635–8.
Kuwano H, Matsuda H, Matsuoka H, Kai H, Okudaira Y, Sugimachi K. Intra-epithelial carcinoma concomitant with esophageal squamous cell carcinoma. Cancer. 1987;59:783–7.
Kuwano H, Ueo H, Sugimachi K, Inokuchi K, Toyoshima S, Enjoji M. Glandular or mucus-secreting components in squamous cell carcinoma of the esophagus. Cancer. 1985;56:514–8.
Kuwano H, Yokobori T, Ide M, Saeki H, Sohda M, Makoto Sakai M, et al. Coexistence of superficial carcinogenesis of resident epithelium besides neuroendocrine neoplasm of the digestive tract. Cancer Med. 2022;11:983–92.
Kuwano H, Miyazaki T, Tsutsumi S, Fukuchi M, Nomoto K-I, Shimura T, et al. Malignant transformation of the mouse anorectal epithelium induced by an inoculated human cancer cell line. Dig Dis Sci. 2004;49:1912–21.
Hu B, Castillo E, Harewood L, Ostano P, Reymond A, Dummer R, et al. Multifocal epithelial tumors and field cancerization from loss of mesenchymal CSL signaling. Cell. 2012;149:1207–20.
Rutter M, Saunders B, Wilkinson K, Rumbles S, Schofield G, Kamm M, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126:451–9.
Saadi A, Shannon NB, Lao-Sirieix P, O’Donovan M, Walker E, Clemons NJ, et al. Stromal genes discriminate preinvasive from invasive disease, predict outcome, and highlight inflammatory pathways in digestive cancers. Proc Natl Acad Sci USA. 2010;107:2177–82.
Fernandez-Sanchez ME, Barbier S, Whitehead J, Béalle G, Michel A, Latorre-Ossa H, et al. Mechanical induction of the tumorigenic beta-catenin pathway by tumour growth pressure. Nature. 2015;523:92–5.
Thirlwell C, Will OC, Domingo E, Graham TA, McDonald SAC, Oukrif D, et al. Clonality assessment and clonal ordering of individual neoplastic crypts shows polyclonality of colorectal adenomas. Gastroenterology. 2010;138:1441–54.
Lin J, Takata M, Murata H, Goto Y, Kido K, Ferrone S, et al. Polyclonality of BRAF mutations in acquired melanocytic nevi. J Natl Cancer Inst. 2009;101:1423–7.
Guccione C, Yadlapati R, Shah S, Knight R, Curtius K. Challenges in determining the role of microbiome evolution in Barrett’s esophagus and progression to esophageal adenocarcinoma. Microorganisms. 2021;9:2003.
Wang X, Undi RB, Ali N, Huycke MM. It takes a village: microbiota, parainflammation, paligenosis and bystander effects in colorectal cancer initiation. Dis Model Mech. 2021;14:dmm048793.
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97.
Boku N. HER2-positive gastric cancer. Gastric Cancer. 2014;17:1–12.
Abrahao-Machado LF, Scapulatempo-Neto C. HER2 testing in gastric cancer: an update. World J Gastroenterol. 2016;22:4619–25.
Satala CB, Jung I, Stefan-van Staden RI, Kovacs Z, Molnar C, Bara T Jr, et al. HER2 heterogeneity in gastric cancer: a comparative study, using two commercial antibodies. J Oncol. 2020;2020:8860174.
Yamashita H, Yando Y, Nishio M, Hamaguchi M, Mita K, Kobayashi S, et al. Immunohistochemical evaluation of hormone receptor status for predicting response to endocrine therapy in metastatic breast cancer. Breast Cancer. 2006;13:74–83.
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–95.
Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805–16.
Early Breast Cancer Trialists' Collaborative G, Davies C, Godwin J, Gray R, Clarke M, Cutter D, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–84.
Klammer H, Mladenov E, Li F, Iliakis G. Bystander effects as manifestation of intercellular communication of DNA damage and of the cellular oxidative status. Cancer Lett. 2015;356:58–71.
Verma N, Tiku AB. Significance and nature of bystander responses induced by various agents. Mutat Res Rev Mutat Res. 2017;773:104–21.
Yamasaki H, Katoh F. Novel method for selective killing of transformed rodent cells through intercellular communication, with possible therapeutic applications. Cancer Res. 1988;48:3203–7.
Aasen T, Mesnil M, Naus CC, Lampe PD, Laird DW. Gap junctions and cancer: communicating for 50 years. Nat Rev Cancer. 2016;16:775–88.
Elshami AA, Saavedra A, Zhang H, Kucharczuk JC, Spray DC, Fishman GI, et al. Gap junctions play a role in the “bystander effect” of the herpes simplex virus thymidine kinase/ganciclovir system in vitro. Gene Ther. 1996;3:85–92.
Arora S, Heyza JR, Chalfin EC, Ruch RJ, Patrick SM. Gap junction intercellular communication positively regulates cisplatin toxicity by inducing DNA damage through bystander signaling. Cancers. 2018;10:368.
Garcia-Rodriguez L, Perez-Torras S, Carrio M, Cascante A, García-Ribas I, Mazo A, et al. Connexin-26 is a key factor mediating gemcitabine bystander effect. Mol Cancer Ther. 2011;10:505–17.
Xiao J, Wang X, Wu Y, Zhao Q, Liu X, Zhang G, et al. Synergistic effect of resveratrol and HSV-TK/GCV therapy on murine hepatoma cells. Cancer Biol Ther. 2019;20:183–91.
Xu Z, Guo D, Jiang Z, Tong R, Jiang P, Bai L, et al. Novel HER2-targeting antibody-drug conjugates of trastuzumab beyond T-DM1 in breast cancer: Trastuzumab deruxtecan(DS-8201a) and (vic-)trastuzumab duocarmazine (SYD985). Eur J Med Chem. 2019;183: 111682.
Ogitani Y, Hagihara K, Oitate M, Naito H, Agatsuma T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016;107:1039–46.
Modi S, Saura C, Yamashita T, Park YH, Kim S-B, Tamura K, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382:610–21.
Shitara K, Iwata H, Takahashi S, Tamura K, Park H, Modi S, et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive gastric cancer: a dose-expansion, phase 1 study. Lancet Oncol. 2019;20:827–36.
Shitara K, Bang YJ, Iwasa S, Sugimoto N, Ryu M-H, Sakai D, et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med. 2020;382:2419–30.
Kubo N, Araki K, Kuwano H, Shirabe K. Cancer-associated fibroblasts in hepatocellular carcinoma. World J Gastroenterol. 2016;22:6841–50.
Fiori ME, Di Franco S, Villanova L, Bianca P, Stassi G, De Maria R. Cancer-associated fibroblasts as abettors of tumor progression at the crossroads of EMT and therapy resistance. Mol Cancer. 2019;18:70.
Bu L, Baba H, Yasuda T, Uchihara T, Ishimoto T. Functional diversity of cancer-associated fibroblasts in modulating drug resistance. Cancer Sci. 2020;111:3468–77.
Baba Y, Nomoto D, Okadome K, Ishimoto T, Iwatsuki M, Miyamoto Y, et al. Tumor immune microenvironment and immune checkpoint inhibitors in esophageal squamous cell carcinoma. Cancer Sci. 2020;111:3132–41.
Bai H, Wang Z, Chen K, Zhao J, Lee JJ, Wang S, et al. Influence of chemotherapy on EGFR mutation status among patients with non-small-cell lung cancer. J Clin Oncol. 2012;30:3077–83.
Koizumi F, Shimoyama T, Taguchi F, Saijo N, Nishio K. Establishment of a human non-small cell lung cancer cell line resistant to gefitinib. Int J Cancer. 2005;116:36–44.
Taguchi F, Koh Y, Koizumi F, Tamura T, Saijo N, Nishio K. Anticancer effects of ZD6474, a VEGF receptor tyrosine kinase inhibitor, in gefitinib (“Iressa”)-sensitive and resistant xenograft models. Cancer Sci. 2004;95:984–9.
Azuma Y, Yokobori T, Mogi A, Yajima T, Kosaka T, Iijima M, et al. Cancer exosomal microRNAs from gefitinib-resistant lung cancer cells cause therapeutic resistance in gefitinib-sensitive cells. Surg Today. 2020;50:1099–106.
Kuwano H, Hashizume M, Yang Y, Kholoussy AM, Matsumoto T. The nature of inner cellular lining of the expanded polytetrafluoroethylene vascular graft: immunohistochemical study. Int Surg. 1992;77:186–9.
Okamoto R, Yajima T, Yamazaki M, Kanai T, Mukai M, Okamoto S, et al. Damaged epithelia regenerated by bone marrow-derived cells in the human gastrointestinal tract. Nat Med. 2002;8:1011–7.
Surinov BP, Karpova NA, Isaeva VG, Kulish Iu S. [Communicative behavioral effects and disorders of immunity]. Zh Vyssh Nerv Deiat Im I P Pavlova. 1998;48:1073–9.
Surinov VP, Karpova NA, Isaeva VG, Kulish Iu S. [Natural excretions of mice in the postradiation period and contact induction of immunodeficiencies]. Radiats Biol Radioecol. 1998;38:9–14.
Mothersill C, Bucking C, Smith RW, Agnihotri N, Oneill A, Kilemade M, et al. Communication of radiation-induced stress or bystander signals between fish in vivo. Environ Sci Technol. 2006;40:6859–64.
Acknowledgements
We thank Ms. Sayaka Okada and Ms. Kayoko Takahashi for their expert assistance.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
We have no conflicts of interest to declare in association with this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kuwano, H., Yokobori, T., Miyazaki, T. et al. Field carcinogenesis and biological significance of the potential of the bystander effect: carcinogenesis, therapeutic response, and tissue regeneration. Surg Today 53, 545–553 (2023). https://doi.org/10.1007/s00595-022-02524-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-022-02524-5