Abstract
Purpose
Decreased irisin levels may be associated with the development of emphysema. Similarly, emphysematous changes may develop in patients with chronic lung allograft dysfunction (CLAD) after living-donor lobar lung transplantation (LDLLT). We investigated the severity of emphysematous changes and the relationship between irisin levels and CLAD after bilateral LDLLT and cadaveric lung transplantation (CLT).
Methods
The subjects of this retrospective study were 59 recipients of bilateral LDLLT (n = 31) or CLT (n = 28), divided into a non-CLAD group (n = 41), a LDLLT-CLAD group (n = 11), and a CLT-CLAD group (n = 7). We compared the severity of emphysematous changes, the skeletal muscle mass, and the plasma irisin levels among the groups.
Results
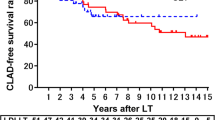
The emphysematous changes were significantly more severe in the LDLLT-CLAD and CLT-CLAD groups (p = 0.046 and 0.036), especially in patients with bronchiolitis obliterans syndrome (BOS), than in the non-CLAD group. Although the skeletal muscle mass was similar in all the groups, the plasma irisin levels were significantly lower in the LDLLT-CLAD group (p = 0.022), especially in the patients with BOS after LDLLT, than in the non-CLAD group.
Conclusion
Emphysematous changes and lower levels of plasma irisin were associated with CLAD, especially in patients with BOS, after bilateral LDLLT.







Similar content being viewed by others
References
Starnes VA, Bowdish ME, Woo MS, Barbers RG, Schenkel FA, Horn MV, et al. A decade of living lobar lung transplantation: recipient outcomes. J Thorac Cardiovasc Surg. 2004;127(1):114–22.
Date H, Sato M, Aoyama A, Yamada T, Mizota T, Kinoshita H, et al. Living-donor lobar lung transplantation provides similar survival to cadaveric lung transplantation even for very ill patientsdagger. Eur J Cardiothorac Surg. 2015;47(6):967–72 (discussion 72-3).
Sugimoto S, Yamamoto H, Kurosaki T, Otani S, Okazaki M, Yamane M, et al. Impact of chronic lung allograft dysfunction, especially restrictive allograft syndrome, on the survival after living-donor lobar lung transplantation compared with cadaveric lung transplantation in adults: a single-center experience. Surg Today. 2019;49(8):686–93.
Yamamoto H, Sugimoto S, Tanaka S, Kurosaki T, Otani S, Yamane M, et al. A single-nucleotide polymorphism in a gene modulating glucocorticoid sensitivity is associated with the decline in total lung capacity after lung transplantation. Surg Today. 2018;49(3):268–74.
Miyamoto E, Chen F, Aoyama A, Sato M, Yamada T, Date H. Unilateral chronic lung allograft dysfunction is a characteristic of bilateral living-donor lobar lung transplantation. Eur J Cardiothorac Surg. 2015;48(3):463–9.
Sugimoto S, Otani S, Ohki T, Kurosaki T, Miyoshi K, Yamane M, et al. Lung retransplantation in an adult 13 years after single lobar transplant in childhood. Gen Thorac Cardiovasc Surg. 2017;65:539–41.
Eberlein M, Permutt S, Chahla MF, Bolukbas S, Nathan SD, Shlobin OA, et al. Lung size mismatch in bilateral lung transplantation is associated with allograft function and bronchiolitis obliterans syndrome. Chest. 2012;141(2):451–60.
Verleden GM, Glanville AR, Lease ED, Fisher AJ, Calabrese F, Corris PA, et al. Chronic lung allograft dysfunction: Definition, diagnostic criteria, and approaches to treatment-A consensus report from the Pulmonary Council of the ISHLT. J Heart Lung Transpl. 2019;38(5):493–503.
Ijiri N, Kanazawa H, Asai K, Watanabe T, Hirata K. Irisin, a newly discovered myokine, is a novel biomarker associated with physical activity in patients with chronic obstructive pulmonary disease. Respirology. 2015;20(4):612–7.
Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463–8.
Timmons JA, Baar K, Davidsen PK, Atherton PJ. Is irisin a human exercise gene? Nature. 2012;488(7413):E9-10 (discussion E-1).
Arhire LI, Mihalache L, Covasa M. Irisin: a hope in understanding and managing obesity and metabolic syndrome. Front Endocrinol. 2019;10:524.
Wang Z, Chen K, Han Y, Zhu H, Zhou X, Tan T, et al. Irisin protects heart against ischemia-reperfusion injury through a SOD2-dependent mitochondria mechanism. J Cardiovasc Pharmacol. 2018;72(6):259–69.
Gonzalez-Gil AM, Elizondo-Montemayor L. The Role of Exercise in the Interplay between Myokines, Hepatokines, Osteokines, Adipokines, and modulation of inflammation for energy substrate redistribution and fat mass loss: a review. Nutrients. 2020;12(6):1899.
Sugiyama Y, Asai K, Yamada K, Kureya Y, Ijiri N, Watanabe T, et al. Decreased levels of irisin, a skeletal muscle cell-derived myokine, are related to emphysema associated with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2017;12:765–72.
Date H, Aoe M, Nagahiro I, Sano Y, Matsubara H, Goto K, et al. How to predict forced vital capacity after living-donor lobar-lung transplantation. J Heart Lung Transpl. 2004;23(5):547–51.
Hirano Y, Sugimoto S, Mano T, Kurosaki T, Miyoshi K, Otani S, et al. Prolonged administration of twice-daily bolus intravenous tacrolimus in the early phase after lung transplantation. Ann Transplant. 2017;22:484–92.
Sugimoto S, Yamane M, Otani S, Kurosaki T, Okahara S, Hikasa Y, et al. Airway complications have a greater impact on the outcomes of living-donor lobar lung transplantation recipients than cadaveric lung transplantation recipients. Surg Today. 2018;48(9):848–55.
Christie JD, Carby M, Bag R, Corris P, Hertz M, Weill D. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction Part II: Definition. A Consensus Statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transpl. 2005;24(10):1454–9.
Glanville AR, Verleden GM, Todd JL, Benden C, Calabrese F, Gottlieb J, et al. Chronic lung allograft dysfunction: Definition and update of restrictive allograft syndrome-A consensus report from the Pulmonary Council of the ISHLT. J Heart Lung Transpl. 2019;38(5):483–92.
Shinya T, Sato S, Kato K, Gobara H, Akaki S, Date H, et al. Assessment of mean transit time in the engrafted lung with 133Xe lung ventilation scintigraphy improves diagnosis of bronchiolitis obliterans syndrome in living-donor lobar lung transplant recipients. Ann Nucl Med. 2008;22(1):31–9.
Yamamoto H, Sugimoto S, Kurosaki T, Miyoshi K, Otani S, Okazaki M, et al. Lung perfusion scintigraphy to detect chronic lung allograft dysfunction after living-donor lobar lung transplantation. Sci Rep. 2020;10(1):10595.
Xie M, Wang W, Dou S, Cui L, Xiao W. Quantitative computed tomography measurements of emphysema for diagnosing asthma-chronic obstructive pulmonary disease overlap syndrome. Int J Chron Obstruct Pulmon Dis. 2016;11:953–61.
Makino Y, Shimada Y, Hagiwara M, Kakihana M, Park J, Kajiwara N, et al. Assessment of emphysema severity as measured on three-dimensional computed tomography images for predicting respiratory complications after lung surgery. Eur J Cardiothorac Surg. 2018;54(4):671–6.
Hamaguchi Y, Kaido T, Okumura S, Kobayashi A, Hammad A, Tamai Y, et al. Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition. 2016;32(11–12):1200–5.
Takenaka T, Yamazaki K, Miura N, Mori R, Takeo S. The prognostic impact of tumor volume in patients with clinical Stage IA non-small cell lung cancer. J Thorac Oncol. 2016;11(7):1074–80.
Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transpl. 2013;48(3):452–8.
Royer PJ, Olivera-Botello G, Koutsokera A, Aubert JD, Bernasconi E, Tissot A, et al. Chronic lung allograft dysfunction: a systematic review of mechanisms. Transplantation. 2016;100(9):1803–14.
Yamane M, Date H, Okazaki M, Toyooka S, Aoe M, Sano Y. Long-term improvement in pulmonary function after living donor lobar lung transplantation. J Heart Lung Transpl. 2007;26(7):687–92.
Hughes VA, Frontera WR, Wood M, Evans WJ, Dallal GE, Roubenoff R, et al. Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci. 2001;56(5):B209–17.
Huh JY, Panagiotou G, Mougios V, Brinkoetter M, Vamvini MT, Schneider BE, et al. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism. 2012;61(12):1725–38.
Nowinska K, Jablonska K, Pawelczyk K, Piotrowska A, Partynska A, Gomulkiewicz A, et al. Expression of Irisin/FNDC5 in cancer cells and stromal fibroblasts of non-small cell lung cancer. Cancers. 2019;11(10):1538.
Qiu S, Cai X, Yin H, Zügel M, Sun Z, Steinacker JM, et al. Association between circulating irisin and insulin resistance in non-diabetic adults: a meta-analysis. Metabolism. 2016;65(6):825–34.
Funding
This work was supported by a Grant-in-Aid for Scientific Research (Grant nos. 19K09305 and 20K1774702) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Toshio Shiotani and his co-authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shiotani, T., Sugimoto, S., Yamamoto, H. et al. Emphysematous changes and lower levels of plasma irisin are associated with bronchiolitis obliterans syndrome after bilateral living-donor lobar lung transplantation. Surg Today 52, 294–305 (2022). https://doi.org/10.1007/s00595-021-02339-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-021-02339-w