Abstract
Background
In addition to the direct power of anticancer drugs, the effectiveness of anticancer therapy depends on the host immune function. The present study investigated whether or not the reduction rate and histological response of preoperative chemotherapy were related to the immune microenvironment surrounding a primary tumor of the rectum.
Methods
Sixty-five patients received preoperative chemotherapy followed by resection from 2012 to 2014; all of these patients were retrospectively analyzed. CD3, CD8, and FoxP3 were immunohistochemically examined as markers for T lymphocytes, cytotoxic T lymphocytes, and regulatory T lymphocytes (Treg), respectively. The correlation between the tumor-infiltrating lymphocyte composition and the tumor reduction rate and histological response to neoadjuvant chemotherapy was investigated.
Results
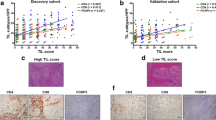
The average tumor reduction rate was 41.5% ± 18.8%. According to RECIST, 47 patients (72.3%) achieved a partial response (PR), and 1 patient (1.5%) achieved a complete response (CR). Eight patients (12.3%) showed a grade 2 histological response, and 2 (3.1%) showed a grade 3 response. A multivariate analysis demonstrated that a low Treg infiltration in stromal cell areas was significantly associated with the achievement of a PR or CR [odds ratio (OR) 7.69; 95% confidence interval (CI) 1.96–33.33; p < 0.01] and a histological grade 2 or 3 response (OR 11.11; 95% CI 1.37–98.04; p = 0.02).
Conclusion
A low Treg infiltration in the stromal cell areas may be a marker of a good response to neoadjuvant chemotherapy in patients with locally advanced rectal cancer.


Similar content being viewed by others
References
Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114–23.
Gerard JP, Conroy T, Bonnetain F, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3–4 rectal cancer: results of FFCD 9203. J Clin Oncol. 2006;24:4620–5.
Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30:1926–33.
Peeters KC, van de Velde CJ, Leer JW, et al. Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: increased bowel dysfunction in irradiated patients—a Dutch colorectal cancer group study. J Clin Oncol. 2005;23:6199–206.
Pollack J, Holm T, Cedermark B, et al. Long-term effect of preoperative radiation therapy on anorectal function. Dis Colon Rectum. 2006;49:345–52.
Birginsson H, Pahlman L, Gunnarsson U, et al. Adverse effects of preoperative radiation therapy for rectal cancer: long-term follow-up of the Swedish Rectal Cancer Trial. J Clin Oncol. 2005;23:8697–705.
Marijnen CA, van de Velde CJ, Putter H, et al. Impact of short-term preoperative radiotherapy on health-related quality of life and sexual functioning in primary rectal cancer: report of a multicenter randomized trial. J Clin Oncol. 2005;23:1847–58.
Stephens RJ, Thompson LC, Quirke P, et al. Impact of short-course preoperative radiotherapy for rectal cancer on patients’ quality of life: date from the Medical Research Council CR07 National Cancer Institute of Canada Clinical Trials Group C016 randomized clinical trial. J Clin Oncol. 2010;28:4233–9.
Cho Y, Miyamoto M, Kato K, et al. CD4+ and CD8+ T cells cooperate to improve prognosis of patients with esophageal squamous cell carcinoma. Cancer Res. 2003;63:1555–9.
Hiraoka K, Miyamoto M, Cho Y, et al. Concurrent infiltration by CD8+ T cells and CD4+ T cells is favourable prognostic factor in non-small-cell lung carcinoma. Br J Cancer. 2006;94:275–80.
Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. J Clin Oncol. 2013;31:860–7.
Zhang L, Conejo-Garcia JR, Katsaris D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–13.
Mei Z, Liu Y, Liu C, et al. Tumour-infiltrating inflammation and prognosis in colorectal cancer: systematic review and meta-analysis. Br J Cancer. 2014;110:1595–605.
Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9.
deLeeuw RJ, Kost SE, Kakal JA, et al. The prognostic value of FoxP3+ tumor-infiltrating lymphocytes in cancer: a critical review of the literature. Clin Cancer Res. 2012;18:3022–9.
Salama P, Phillips M, Grieu F, et al. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27:186–92.
Nowak AK, Lake RA, Marzo AL, et al. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J Immunol. 2003;170:4905–13.
Ghiringhelli F, Menard C, Puig PE, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–8.
Zhang B, Bowerman NA, Salama JK, et al. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Exp Med. 2007;204:49–55.
Ramakrishman R, Assudani D, Nagaraj S, et al. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J Clin invest. 2010;120:1111–24.
Burnette BC, Liang H, Lee Y, et al. The efficacy of radiotherapy relies upon induction of type I interferon-dependent innate and adaptive immunity. Cancer Res. 2011;71:2488–96.
Gupta A, Probst HC, Vuong V, et al. Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. J Immunol. 2012;189:558–66.
Kim S, Ramakrishman R, Lavilla-Alonso S, et al. Radiation-induced autophagy potentiates immunotherapy of cancer via up-regulation of mannose 6-phosphate receptor on tumor cells in mice. Cancer Immunol Immunother. 2014;63:1009–21.
Frey B, Stache C, Rubner Y, et al. Combined treatment of human colorectal tumor cell lines with chemotherapeutic agents and ionizing irradiation can in vitro induce tumor cell death forms with immunogenic potential. J Immunotoxicol. 2012;9:301–13.
Maeda K, Hazama S, Tokuno K, et al. Impact of chemotherapy for colorectal cancer on regulatory T-cells and tumor immunity. Anticancer Res. 2011;31:4569–74.
Ladoire S, Arnould L, Apetoh L, et al. Pathological complete response to neoadjuvant chemotherapy of breast carcinoma is associated with the disappearance of tumor-infiltrating foxp3+ regulatory T cells. Clin Cancer Res. 2008;14:2413–20.
McCoy MJ, Hemmings C, Anyaegbu CC, et al. Tumour-infiltrating regulatory T cell density before neoadjuvant chemoradiotherapy for rectal cancer does not predict treatment response. Oncotarget. 2017;8:19803–13.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47.
Homma Y, Taniguchi K, Murakami T, et al. Immunological impact of neoadjuvant chemoradiotherapy in patients with borderline resectable pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2014;21(2):670–6.
Nakagawa K, Tanaka K, Homma Y, et al. Low Infiltration of peritumoral regulatory T cell predicts worse outcome following resection of colorectal liver metastases. Ann Surg Oncol. 2015;22(1):180–6.
McCoy MJ, Hemmings C, Miller TJ, et al. Low stromal Foxp3+ regulatory T-cell density is associated with complete response to neoadjuvant chemoradiotherapy in rectal cancer. Br J Cancer. 2015;113:1677–86.
Teng F, Meng X, Kong L, et al. Tumor-infiltrating lymphocytes, forkhead box P3, programmed death ligand-1, and cytotoxic T lymphocyte-associated antigen-4 expressions before and after neoadjuvant chemoradiation in rectal cancer. Transl Res. 2015;166:721–32.
Matsutani S, Shibutani M, Maeda K, et al. Significance of tumor-infiltrating lymphocytes before and after neoadjuvant therapy for rectal cancer. Cancer Sci. 2018;109:966–79.
Roxburgh CS, Shia J, Vakiani E, Daniel T, Weiser MR. Potential immune priming of the tumor microenvironment with FOLFOX chemotherapy in locally advanced rectal cancer. Oncoimminology. 2018;22:e1435227.
Rettig L, Seidenberg S, Parvanova I, et al. Gemcitabine depletes regulatory T-cells in human and mice and enhances triggering of vaccine-specific cytotoxic T-cells. Int J Cancer. 2011;129:832–8.
van der Most RG, Currie AJ, Mahendran S, et al. Tumor eradication after cyclophosphamide depends on concurrent depletion of regulatory T cells: a role for cycling TNFR2-expressing effector-suppressor T cells in limiting effective chemotherapy. Cancer Immunol Immunother. 2009;58:1219–28.
Frey B, Rubner Y, Kulzer L, et al. Antitumor immune responses induced by ionizing irradiation and further immune stimulation. Cancer Immunol Immunother. 2014;63:29–36.
Rizzo S, Bronte G, Fanale D, et al. Prognostic vs. predictive molecular biomarkers in colorectal cancer: is KRAS and BRAF wild type status required for anti-EGFR therapy? Cancer Treat Rev. 2010;36(3):S56-61.
Vakiani E, Solit DB. KRAS and BRAF: drug targets and predictive biomarkers. J Pathol. 2011;223:219–29.
Granville CA, Memmott RM, Balogh A, et al. A central role for Foxp+ regulatory T cell in K-Ras-driven lung tumorigenesis. PLoS ONE. 2009;4:5061.
Zdanov S, Mandapathil M, Abu Eid R, et al. Mutant KRAS conversion of conventional T cells into regulatory T cells. Cancer Immunol Res. 2016;4:354–65.
Rödel C, Thomas P, Papadoupolos T, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005;23:8688–96.
Sclafani F, Cunningham D. Neoadjuvant chemotherapy without radiotherapy for locally advanced rectal cancer. Fut Oncol. 2014;10:2243–57.
Michael-Robinson JM, Biemer-Hüttmann A, Purdie DM, et al. Tumour infiltrating lymphocytes and apoptosis are independent features in colorectal cancer stratified according to microsatellite instability status. Gut. 2001;48:360–6.
Yoshino T, Arnold D, Taniguchi H, et al. Pan-Asian adapted ESMO consensus guidelines for management of patients with metastatic colorectal cancer: a JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann oncol. 2018;29:44–70.
Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–422.
Acknowledgements
We would like to give many thanks to H. Sakurada for performing skillful laboratory work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest for this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sekizawa, K., Nakagawa, K., Ichikawa, Y. et al. Relationship between stromal regulatory T cells and the response to neoadjuvant chemotherapy for locally advanced rectal cancer. Surg Today 52, 198–206 (2022). https://doi.org/10.1007/s00595-021-02311-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-021-02311-8