Abstract
Purpose
Gastric cancer (GC) is a common malignancy, especially in East Asian countries. There is emerging evidence that circulating neutrophil and platelet levels correlate with cancer progression. We evaluated the short- and long-term outcomes of GC patients systemically, to compare the original neutrophil–platelet score (NPS) and our modified NPS (mNPS).
Methods
We analyzed the original pre-operative NPS and the mNPS of 621 GC patients.
Results
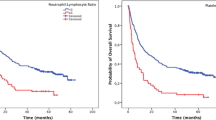
Racial differences between the United Kingdom and East Asian countries accounted for compelling deviation in classification using the original NPS, which could not reliably stratify the prognoses of Japanese GC patients. We developed the mNPS using appropriate cutoff levels for pre-operative neutrophils and platelets, and demonstrated that the pre-operative mNPS was significantly correlated with all of the well-established clinicopathological factors for disease development, including advanced T stage, venous and lymphatic vessel invasion, lymph node/peritoneal /distant metastasis, and tumor-node-metastasis stage. The pre-operative mNPS could stratify prognostication for both overall survival (OS) and disease-free survival (DFS): a high pre-operative mNPS was an independent prognostic factor for the OS and DFS of GC patients and also an independent predictor of post-operative surgical site infection after gastrectomy.
Conclusion
Calculating the mNPS could help clinicians to stratify the surgical and oncological risks of patients with GC.

Similar content being viewed by others
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics. 2012. CA Cancer J Clin. 2015;65(2):87–108.
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386386.
Andre N, Schmiegel W. Chemoradiotherapy for colorectal cancer. Gut. 2005;54(8):1194–202.
Lurje G, Zhang W, Lenz HJ. Molecular prognostic markers in locally advanced colon cancer. Clin Colorectal Cancer. 2007;6(10):683–90.
Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–44.
Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet (Lond Engl). 2001;357(9255):539–45.
Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073–81.
Mohri Y, Tanaka K, Toiyama Y, Ohi M, Yasuda H, Inoue Y, et al. Impact of preoperative neutrophil to lymphocyte ratio and postoperative infectious complications on survival after curative gastrectomy for gastric cancer: a single institutional cohort study. Medicine. 2016;95(11):e3125.
Toiyama Y, Shimura T, Yasuda H, Fujikawa H, Okita Y, Kobayashi M, et al. Clinical burden of C-reactive protein/albumin ratio before curative surgery for patients with gastric cancer. Anticancer Res. 2016;36(12):6491–8.
Ide S, Toiyama Y, Okugawa Y, Oki S, Yasuda H, Fujikawa H, et al. Clinical significance of C-reactive protein-to-albumin ratio with rectal cancer patient undergoing chemoradiotherapy followed by surgery. Anticancer Res. 2017;37(10):5797–804.
Okugawa Y, Toiyama Y, Oki S, Ide S, Yamamoto A, Ichikawa T, et al. Feasibility of assessing prognostic nutrition index in patients with rectal cancer who receive preoperative chemoradiotherapy. JPEN J Parenter Enteral Nutr. 2018;42(6):998–1007.
Okugawa Y, Shirai Y, Toiyama Y, Saigusa S, Hishida A, Yokoe T, et al. Clinical burden of modified glasgow prognostic scale in colorectal cancer. Anticancer Res. 2018;38(3):1599–610.
Sreeramkumar V, Adrover JM, Ballesteros I, Cuartero MI, Rossaint J, Bilbao I, et al. Neutrophils scan for activated platelets to initiate inflammation. Science (NY NY). 2014;346(6214):1234–8.
Watt DG, Proctor MJ, Park JH, Horgan PG, McMillan DC. The neutrophil-platelet score (NPS) predicts survival in primary operable colorectal cancer and a variety of common cancers. PLoS ONE. 2015;10(11):e0142159.
Koike Y, Miki C, Okugawa Y, Yokoe T, Toiyama Y, Tanaka K, et al. Preoperative C-reactive protein as a prognostic and therapeutic marker for colorectal cancer. J Surg Oncol. 2008;98(7):540–4.
Shirai Y, Okugawa Y, Hishida A, Ogawa A, Okamoto K, Shintani M, et al. Fish oil-enriched nutrition combined with systemic chemotherapy for gastrointestinal cancer patients with cancer cachexia. Sci Rep. 2017;7(1):4826.
Oki S, Toiyama Y, Okugawa Y, Shimura T, Okigami M, Yasuda H, et al. Clinical burden of preoperative albumin-globulin ratio in esophageal cancer patients. Am J Surg. 2017;214(5):891–8.
Fujikawa H, Toiyama Y, Inoue Y, Imaoka H, Shimura T, Okigami M, et al. Prognostic impact of preoperative albumin-to-globulin ratio in patients with colon cancer undergoing surgery with curative intent. Anticancer Res. 2017;37(3):1335–422.
Shimura T, Toiyama Y, Saigusa S, Imaoka H, Okigami M, Fujikawa H, et al. Inflammation-based prognostic scores as indicators to select candidates for primary site resection followed by multimodal therapy among colorectal cancer patients with multiple metastases. Int J Clin Oncol. 2017;22(4):758–66.
Mori K, Toiyama Y, Saigusa S, Fujikawa H, Hiro J, Kobayashi M, et al. Systemic analysis of predictive biomarkers for recurrence in colorectal cancer patients treated with curative surgery. Dig Dis Sci. 2015;60(8):2477–87.
Shaul ME, Fridlender ZG. Cancer related circulating and tumor-associated neutrophils—subtypes, sources and function. FEBS J. 2018.
Schmidt H, Bastholt L, Geertsen P, Christensen IJ, Larsen S, Gehl J, et al. Elevated neutrophil and monocyte counts in peripheral blood are associated with poor survival in patients with metastatic melanoma: a prognostic model. Br J Cancer. 2005;93(3):273–8.
Lechner MG, Liebertz DJ, Epstein AL. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J Immunol (Baltimore, Md : 1950). 2010;185(4):2273–84.
Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111(10):4902–7.
Demers M, Wagner DD. NETosis: a new factor in tumor progression and cancer-associated thrombosis. Semin Thromb Hemost. 2014;40(3):277–83.
Demers M, Krause DS, Schatzberg D, Martinod K, Voorhees JR, Fuchs TA, et al. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc Natl Acad Sci USA. 2012;109(32):13076–81.
Bystricky B, Reuben JM, Mego M. Circulating tumor cells and coagulation-minireview. Crit Rev Oncol Hematol. 2017;114:33–42.
Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science (NY, NY). 2004;303(5663):1532–5.
Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Investig. 2013.
Naschitz JE, Yeshurun D, Eldar S, Lev LM. Diagnosis of cancer-associated vascular disorders. Cancer. 1996;77(9):1759–67.
Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Kubota K. Preoperative thrombocytosis is associated with survival after surgery for colorectal cancer. J Surg Oncol. 2012;106(7):887–91.
Martinod K, Wagner DD. Thrombosis: tangled up in NETs. Blood. 2014;123(18):2768–76.
Watt DG, Martin JC, Park JH, Horgan PG, McMillan DC. Neutrophil count is the most important prognostic component of the differential white cell count in patients undergoing elective surgery for colorectal cancer. Am J Surg. 2015;210(1):24–30.
Leitch EF, Chakrabarti M, Crozier JE, McKee RF, Anderson JH, Horgan PG, et al. Comparison of the prognostic value of selected markers of the systemic inflammatory response in patients with colorectal cancer. Br J Cancer. 2007;97(9):1266–70.
Khera A, McGuire DK, Murphy SA, Stanek HG, Das SR, Vongpatanasin W, et al. Race and gender differences in C-reactive protein levels. J Am Coll Cardiol. 2005;46(3):464–9.
Yamada S, Gotoh T, Nakashima Y, Kayaba K, Ishikawa S, Nago N, et al. Distribution of serum C-reactive protein and its association with atherosclerotic risk factors in a Japanese population: Jichi Medical School Cohort Study. Am J Epidemiol. 2001;153(12):1183–90.
Lakoski SG, Cushman M, Palmas W, Blumenthal R, D'Agostino RB Jr, Herrington DM. The relationship between blood pressure and C-reactive protein in the multi-ethnic study of atherosclerosis (MESA). J Am Coll Cardiol. 2005;46(10):1869–74.
McDade TW, Rutherford JN, Adair L, Kuzawa C. Population differences in associations between C-reactive protein concentration and adiposity: comparison of young adults in the Philippines and the United States. Am J Clin Nutr. 2009;89(4):1237–45.
Moyes LH, Leitch EF, McKee RF, Anderson JH, Horgan PG, McMillan DC. Preoperative systemic inflammation predicts postoperative infectious complications in patients undergoing curative resection for colorectal cancer. Br J Cancer. 2009;100(8):1236–9.
Canna K, McArdle PA, McMillan DC, McNicol AM, Smith GW, McKee RF, et al. The relationship between tumour T-lymphocyte infiltration, the systemic inflammatory response and survival in patients undergoing curative resection for colorectal cancer. Br J Cancer. 2005;92(4):651–4.
Funding
This study was supported by a research grant from Japanese Society for Palliative Medicine and Mie Medical Research Foundation.
Author information
Authors and Affiliations
Contributions
Study concept and design: YO, YT, MO, KM; provision of samples: AY, YO, KK, CY, SI, TK, HF, HY, JH, SY, MO, TA; acquisition of data: YO, YT, AY, YO, KK, CY, SI, TK, YK, HF, HY, JH, SY, MO, TA; analysis and interpretation of data: YO, YT, MO, TA; statistical analysis: YO, YT; and drafting of the manuscript: YO, YT, MO, MK.
Corresponding authors
Ethics declarations
Conflict of interests
We have no conflict of interest to disclose for this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
595_2019_1873_MOESM2_ESM.tif
Supplementary Fig. 1. Prognostic impact of the modified NPS status divided by stage classification for the overall survival (OS) and disease-free survival (DFS) of patients with gastric cancer (GC). Although prognostic stratification of the mNPS was not significantly different in the population with Stage I or Stage II/III disease, its impact was revealed more clearly in Stage IV GC patients (P=0.04, log rank test) (TIFF 797 kb)
Rights and permissions
About this article
Cite this article
Okugawa, Y., Toiyama, Y., Yamamoto, A. et al. Modified neutrophil-platelet score as a promising marker for stratified surgical and oncological outcomes of patients with gastric cancer. Surg Today 50, 223–231 (2020). https://doi.org/10.1007/s00595-019-01873-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-019-01873-y