Abstract
Background
Although patients with metastatic colorectal cancer (CRC) are often unable to undergo treatment after resection of primary tumors, identifying such patients before surgery is not easy. In this study, we evaluated the association among clinicopathological findings, survival outcomes, and ability to undergo multimodal therapy after primary tumor resection in patients with Stage IV CRC.
Methods
We collected clinicopathological findings and preoperative laboratory data, including carcinoembryonic antigen (CEA) and systemic inflammatory response markers for 92 patients who were treated for Stage IV CRC between 2005 and 2014. We used multivariate analysis on factors that affect prognosis and ability to undergo postoperative treatment.
Results
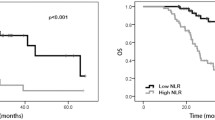
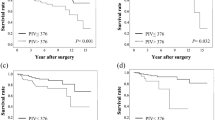
Postoperative multimodal therapy improved overall survival (OS) significantly. Among serum markers, elevated CEA, neutrophil-to-lymphocyte ratio, and modified Glasgow prognosis score (mGPS) were significant indicators of shorter OS. In multivariate analysis, low performance status (P = 0.003), undifferentiated histology type (P = 0.019), and elevated mGPS (P = 0.042) were independent predictors of worse prognosis; and older age (P = 0.016), right-sided colon cancer (P = 0.043), and elevated mGPS (P = 0.031) were independent risk factors for difficulty of introducing postoperative multimodal therapy.
Conclusions
Preoperative mGPS is a useful objective indicator for CRC patients with multiple metastases who are able to undergo primary site resection followed by postoperative multimodal therapy.




Similar content being viewed by others
References
Ferlay J, Soerjomataram I, Dikshit R et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359–E386
Siegel R, Naishadham D, Jemal A (2012) Cancer statistics, 2012. CA Cancer J Clin 62:10–29
Cook AD, Single R, McCahill LE (2005) Surgical resection of primary tumors in patients who present with stage IV colorectal cancer: an analysis of surveillance, epidemiology, and end results data, 1988 to 2000. Ann Surg Oncol 12:637–645
van der Pool AE, Damhuis RA, Ijzermans JN et al (2012) Trends in incidence, treatment and survival of patients with stage IV colorectal cancer: a population-based series. Colorectal Dis 14:56–61
van der Geest LG, Lam-Boer J, Koopman M et al (2015) Nationwide trends in incidence, treatment and survival of colorectal cancer patients with synchronous metastases. Clin Exp Metastasis 32:457–465
Cirocchi R, Trastulli S, Abraha I et al (2012) Non-resection versus resection for an asymptomatic primary tumour in patients with unresectable stage IV colorectal cancer. Cochrane Database Syst Rev 8(8):CD008997. doi:10.1002/14651858.CD008997.pub2
Rahbari NN, Lordick F, Fink C et al (2012) Resection of the primary tumour versus no resection prior to systemic therapy in patients with colon cancer and synchronous unresectable metastases (UICC stage IV): SYNCHRONOUS–a randomised controlled multicentre trial (ISRCTN30964555). BMC Cancer 12:142–151
Lam-Boer JT, Van der Geest LG, Verhoef C et al (2016) Palliative resection of the primary tumor is associated with improved overall survival in incurable stage IV colorectal cancer: a nationwide population-based propensity-score adjusted study in the Netherlands. Int J Cancer 139:2082–2094
Faron M, Pignon JP, Malka D et al (2015) Is primary tumour resection associated with survival improvement in patients with colorectal cancer and unresectable synchronous metastases? A pooled analysis of individual data from four randomised trials. Eur J Cancer 51:166–176
Ahmed S, Leis A, Chandra-Kanthan S et al (2016) Surgical management of the primary tumor in stage iv colorectal cancer: a confirmatory retrospective cohort study. J Cancer 7:837–845
Ahmed S, Leis A, Fields A et al (2014) Survival impact of surgical resection of primary tumor in patients with stage IV colorectal cancer: results from a large population-based cohort study. Cancer 120:683–691
Wilkinson KJ, Chua W, Ng W et al (2010) Management of asymptomatic primary tumours in stage IV colorectal cancer: review of outcomes. World J Gastrointest Oncol 7:513–523
Stillwell AP, Buettner PG, Ho YH (2010) Meta-analysis of survival of patients with stage IV colorectal cancer managed with surgical resection versus chemotherapy alone. World J Surg 34:797–807
Watanabe T, Itabashi M, Shimada Y et al (2015) Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol 20:207–239
Uratani R, Toiyama Y, Shimura T et al (2015) Preoperative lower body mass index correlates with poorer prognosis in patients undergoing curative laparoscopic surgery for colorectal cancer. Anticancer Res 35:5639–5648
Toiyama Y, Hiro J, Shimura T et al (2016) The impact of body mass index on oncological outcomes in colorectal cancer patients with curative intent. Int J Clin Oncol 21(6):1102–1110
Toiyama Y, Inoue Y, Kawamura M et al (2015) Elevated platelet count as predictor of recurrence in rectal cancer patients undergoing preoperative chemoradiotherapy followed by surgery. Int Surg 100:199–207
Mori K, Toiyama Y, Saigusa S et al (2015) Systemic analysis of predictive biomarkers for recurrence in colorectal cancer patients treated with curative surgery. Dig Dis Sci 60:2477–2487
Inoue Y, Iwata T, Okugawa Y et al (2013) Prognostic significance of a systemic inflammatory response in patients undergoing multimodality therapy for advanced colorectal cancer. Oncology 84:100–107
Toiyama Y, Miki C, Inoue Y et al (2011) Evaluation of an inflammation-based prognostic score for the identification of patients requiring postoperative adjuvant chemotherapy for stage II colorectal cancer. Exp Ther Med 2:95–101
Hu H, Krasinskas A, Willis J (2011) Perspectives on current tumor-node-metastasis (TNM) staging of cancers of the colon and rectum. Semin Oncol 38:500–510
Oken MM, Creech RH, Tormey DC et al (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5:649–655
McMillan DC (2009) Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care 12:223–226
Chiang SF, Hung HY, Tang R et al (2012) Can neutrophil-to-lymphocyte ratio predict the survival of colorectal cancer patients who have received curative surgery electively? Int J Colorectal Dis 27:1347–1357
Kwon HC, Kim SH, Oh SY et al (2012) Clinical significance of preoperative neutrophil-lymphocyte versus platelet-lymphocyte ratio in patients with operable colorectal cancer. Biomarkers 17:216–222
Ishizuka M, Nagata H, Takagi K et al (2012) Preoperative thrombocytosis is associated with survival after surgery for colorectal cancer. J Surg Oncol 106:887–891
Damjanov N, Weiss J, Haller DG (2009) Resection of the primary colorectal cancer is not necessary in nonobstructed patients with metastatic disease. Oncologist 14:963–969
McMillan DC (2013) The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev 39:534–540
Proctor MJ, Morrison DS, Talwar D et al (2011) A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer 47:2633–2641
Canna K, McArdle PA, McMillan DC et al (2005) The relationship between tumour T-lymphocyte infiltration, the systemic inflammatory response and survival in patients undergoing curative resection for colorectal cancer. Br J Cancer 92:651–654
McMillan DC, Canna K, McArdle CS (2003) Systemic inflammatory response predicts survival following curative resection of colorectal cancer. Br J Surg 90:215–219
Miki C, Konishi N, Ojima E et al (2004) C-reactive protein as a prognostic variable that reflects uncontrolled up-regulation of the IL-1-IL-6 network system in colorectal carcinoma. Dig Dis Sci 49:970–976
Delmore G (1997) Assessment of nutritional status in cancer patients: widely neglected? Support Care Cancer 5:376–380
McMillan DC, Scott HR, Watson WS et al (1998) Longitudinal study of body cell mass depletion and the inflammatory response in cancer patients. Nutr Cancer 31:101–105
McMillan DC, Watson WS, O’Gorman P et al (2001) Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutr Cancer 39:210–213
McMillan DC (2008) An inflammation-based prognostic score and its role in the nutrition-based management of patients with cancer. Proc Nutr Soc 67:257–262
McMillan DC, Crozier JE, Canna K et al (2007) Evaluation of an inflammation-based prognostic score (GPS) in patients undergoing resection for colon and rectal cancer. Int J Colorectal Dis 22:881–886
Ishizuka M, Nagata H, Takagi K et al (2007) Inflammation-based prognostic score is a novel predictor of postoperative outcome in patients with colorectal cancer. Ann Surg 246:1047–1051
Ishizuka M, Nagata H, Takagi K et al (2013) Inflammation-based prognostic system predicts survival after surgery for stage IV colorectal cancer. Am J Surg 205:22–28
Kishiki T, Masaki T, Matsuoka H et al (2013) Modified Glasgow prognostic score in patients with incurable stage IV colorectal cancer. Am J Surg 206:234–240
Maeda K, Shibutani M, Otani H et al (2013) Prognostic value of preoperative inflammation-based prognostic scores in patients with stage IV colorectal cancer who undergo palliative resection of asymptomatic primary tumors. Anticancer Res 33:5567–5573
Adachi T, Hinoi T, Hattori M et al (2015) The modified Glasgow prognostic score for early mortality in patients with synchronous peritoneal carcinomatosis from colorectal cancer. Surg Today 45:1396–1403
Kobayashi S, Karube Y, Nishihira M et al (2016) Usefulness of inflammation-based prognostic score in patients undergoing lung metastasectomy for colorectal carcinoma. World J Surg 40:1632–1637
Shibutani M, Maeda K, Nagahara H et al (2015) Significance of markers of systemic inflammation for predicting survival and chemotherapeutic outcomes and monitoring tumor progression in patients with unresectable metastatic colorectal cancer. Anticancer Res 35:5037–5046
Kuijpers CC, Sluijter CE, von der Thusen JH et al (2016) Interlaboratory variability in the histologic grading of colorectal adenocarcinomas in a nationwide cohort. Am J Surg Pathol 40:1100–1108
Petrelli F, Tomasello G, Borgonovo K et al (2016) Prognostic survival associated with left-sided vs right-sided colon cancer: a systematic review and meta-analysis. JAMA Oncol. doi:10.1001/jamaoncol.2016.4227
Yahagi M, Okabayashi K, Hasegawa H et al (2016) The worse prognosis of right-sided compared with left-sided colon cancers: a systematic review and meta-analysis. J Gastrointest Surg 20:648–655
Nitsche U, Stogbauer F, Spath C et al (2016) Right sided colon cancer as a distinct histopathological subtype with reduced prognosis. Dig Surg 33:157–163
Ishihara S, Nishikawa T, Tanaka T et al (2014) Prognostic impact of tumor location in stage IV colon cancer: a propensity score analysis in a multicenter study. Int J Surg 12:925–930
Chan M, Hugh-Yeun K, Gresham G et al (2016) Population-based patterns and factors associated with underuse of palliative systemic therapy in elderly patients with metastatic colon cancer. Clin Colorectal Cancer. doi:10.1016/j.clcc.2016.08.004
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10147_2017_1113_MOESM1_ESM.ppt
Supplemental Figure 1 Modified GPS score against histological differentiation. The mGPS2 group had a significantly higher percentage of poorly differentiated histological type (7/20, 35%) than the mGPS0/1 group (6/72, 8.3%, P = 0.0054). Supplemental Figure 2 Histological differentiation against NLR values. The poor histology group had significantly higher median preoperative NLR (5.53, range 0.96–17.71) than the well/moderate histology group (2.98, range 0.57–30.13; P = 0.0332). Supplemental Figure 3 Patient age in relation to whether or not they underwent postoperative treatment. Median age in the untreated group (75 years; range 59–90 years) was significantly older than in the treated group (63 years, range 31–84 years; P = 0.0002) (PPT 194 kb)
About this article
Cite this article
Shimura, T., Toiyama, Y., Saigusa, S. et al. Inflammation-based prognostic scores as indicators to select candidates for primary site resection followed by multimodal therapy among colorectal cancer patients with multiple metastases. Int J Clin Oncol 22, 758–766 (2017). https://doi.org/10.1007/s10147-017-1113-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-017-1113-2