Abstract
Purpose
We reported previously a phase II study of adjuvant chemotherapy consisting of four cycles of vinorelbine (25 mg/m2) and cisplatin (40 mg/m2), given on days 1 and 8, every 4 weeks, to Japanese patients with completely resected stage II or III non-small cell lung cancer (NSCLC; UMIN 000005055). However, the follow-up was too short for us to evaluate a definitive 5-year overall survival rate and after-effects.
Methods
Between December 2006 and January 2011, 60 patients were enrolled in this study. We analyzed relapse-free and overall survival, long-lasting adverse effects, the influence of treatment on recurrent tumors, and the development of a second primary cancer, in relation with the regimen.
Results
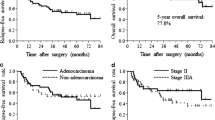
After a median follow-up period of 95.8 months, the 5-year relapse-free and overall survival rates were 51.7 and 76.7%, respectively. Neuralgia developed in one patient and this was the only case of a long-lasting adverse effect. Recurrence developed in 31 patients, 29 of whom received intensive treatment. Although 16 s (or more) primary neoplasms developed among 13 patients, these were common carcinomas in Japan and did not include sarcoma or hematologic malignancies.
Conclusion
Adjuvant vinorelbine and cisplatin chemotherapy showed encouraging relapse-free and overall survival rates, and long-term safety in Japanese patients with resected NSCLC.



Similar content being viewed by others
References
Sawabata N, Miyaoka E, Asamura H, Nakanishi Y, Eguchi K, Mori M, et al. Japanese Joint Committee for Lung Cancer Registration. Japanese lung cancer registry study of 11,663 surgical cases in 2004: demographic and prognosis changes over decade. J Thorac Oncol. 2011;6:1229–35.
Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J, International Adjuvant Lung Cancer Trial Collaborative Group. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004; 350:351–60.
Winton T, Livingston R, Johnson D, Rigas J, Johnston M, Butts C, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352:2589–97.
Douillard JY, Rosell R, De Lena M, Carpagnano F, Ramlau R, Gonzáles-Larriba JL, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006;7:719–27.
Pignon JP, Tribodet H, Scagliotti GV, Douillard JY, Shepherd FA, Stephens RJ, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26:3552–9.
Douillard JY, Tribodet H, Aubert D, Shepherd FA, Rosell R, Ding K, et al. Adjuvant cisplatin and vinorelbine for completely resected non-small cell lung cancer: subgroup analysis of the Lung Adjuvant Cisplatin Evaluation. J Thorac Oncol. 2010;5:220–8.
Wada H, Hitomi S, Teramatsu T. Adjuvant chemotherapy after complete resection in non-small-cell lung cancer. West Japan Study Group for Lung Cancer Surgery. J Clin Oncol. 1996;14:1048–54.
Tada H, Tsuchiya R, Ichinose Y, Koike T, Nishizawa N, Nagai K, et al. A randomized trial comparing adjuvant chemotherapy versus surgery alone for completely resected pN2 non-small cell lung cancer (JCOG9304). Lung Cancer. 2004;43:167–73.
Sonobe M, Okubo K, Teramukai S, Yanagihara K, Sato M, Sato T, et al. Phase II study of adjuvant vinorelbine and cisplatin in Japanese patients with completely resected stage II and III non-small cell lung cancer. Cancer Chemother Pharmacol. 2014;74:1199–206.
Arriagada R, Dunant A, Pignon JP, Bergman B, Chabowski M, Grunenwald D, et al. Long-term results of the international adjuvant lung cancer trial evaluating adjuvant Cisplatin-based chemotherapy in resected lung cancer. J Clin Oncol. 2010;28:35–42.
Mountain CF. Revisions in the international system for staging lung cancer. Chest. 1997;111:1710–17.
Antakli T, Schaefer RF, Rutherford JE, Read RC. Second primary lung cancer. Ann Thorac Surg. 1995;59:863–6.
Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706–14.
Kenmotsu H, Ohde Y, Wakuda K, Nakashima K, Omori S, Ono A, et al. Survival data for postoperative adjuvant chemotherapy comprising cisplatin plus vinorelbine after complete resection of non-small cell lung cancer. Cancer Chemother Pharmacol. 2017. https://doi.org/10.1007/s00280-017-3400-z.
Shukuya T, Takahashi T, Tamiya A, Ono A, Igawa S, Tsuya A, et al. Evaluation of the safety and compliance of 3-week cycles of vinorelbine on days 1 and 8 and cisplatin on day 1 as adjuvant chemotherapy in Japanese patients with completely resected pathological stage IB to IIIA non-small cell lung cancer: a retrospective study. Jpn J Clin Oncol. 2009;39:158–62.
Sonobe M, Nakagawa M, Takenaka K, Katakura H, Adachi M, Yanagihara K, et al. Influence of epidermal growth factor receptor (EGFR) gene mutations on the expression of EGFR, phosphoryl-Akt, and phosphoryl-MAPK, and on the prognosis of patients with non-small cell lung cancer. J Surg Oncol. 2007;95:63–9.
Sonobe M, Kobayashi M, Ishikawa M, Kikuchi R, Nakayama E, Takahashi T, et al. Impact of KRAS and EGFR gene mutations on recurrence and survival in patients with surgically resected lung adenocarcinomas. Ann Surg Oncol. 2012;19(Suppl 3):S347-54.
Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–8.
Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8.
Yokoyama Y, Sonobe M, Yamada T, Sato M, Menju T, Aoyama A, et al. Gefitinib treatment in patients with postoperative recurrent non-small-cell lung cancer harboring epidermal growth factor receptor gene mutations. Int J Clin Oncol. 2015;20:1122–9.
Niibe Y, Hayakawa K. Oligometastases and oligo-recurrence: the new era of cancer therapy. Jpn J Clin Oncol. 2010;40:107–11.
Yano T, Haro A, Yoshida T, Morodomi Y, Ito K, Shikada Y, et al. Prognostic impact of local treatment against postoperative oligometastases in non-small cell lung cancer. J Surg Oncol. 2010;102:852–5.
Sonobe M, Yamada T, Sato M, Menju T, Aoyama A, Sato T, et al. Identification of subsets of patients with favorable prognosis after recurrence in completely resected non-small cell lung cancer. Ann Surg Oncol. 2014;21:2546–54.
Iwamoto Y, Mitsudomi T, Sakai K, Yamanaka T, Yoshioka H, Takahama M, et al. Randomized phase II study of adjuvant chemotherapy with long-term S-1 versus cisplatin + S-1 in completely resected stage II–IIIA non-small cell lung cancer. Clin Cancer Res. 2015;21:5245–52.
Yamamoto N, Kenmotsu H, Yamanaka T, Nakamura S, Tsuboi M. Randomized phase iii study of cisplatin with pemetrexed and cisplatin with vinorelbine for completely resected nonsquamous non-small-cell lung cancer: the JIPANG study protocol. Clin Lung Cancer. 2017. https://doi.org/10.1016/j.cllc.2017.05.020.
Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. J Peripher Nerv Syst. 2008;13:27–46.
Rotolo F, Dunant A, Le Chevalier T, Pignon JP, Arriagada R. IALT Collaborative Group. Adjuvant cisplatin-based chemotherapy in nonsmall-cell lung cancer: new insights into the effect on failure type via a multistate approach. Ann Oncol. 2014; 25:2162–6.
The Japan Lung Cancer Society. 2014. https://www.haigan.gr.jp/guideline/2014/2/140002060100.html. Accessed Aug 2017.
Travis LB, Fosså SD, Schonfeld SJ, McMaster ML, Lynch CF, Storm H, et al. Second cancers among 40,576 testicular cancer patients: focus on long-term survivors. J Natl Cancer Inst. 2005;97:1354–65.
van den Belt-Dusebout AW, de Wit R, Gietema JA, Horenblas S, Louwman MW, Ribot JG, et al. Treatment-specific risks of second malignancies and cardiovascular disease in 5-year survivors of testicular cancer. J Clin Oncol. 2007;25:4370–8.
Johnson BE. Second lung cancers in patients after treatment for an initial lung cancer. J Natl Cancer Inst. 1998;90:1335–45.
Surapaneni R, Singh P, Rajagopalan K, Hageboutros A. Stage I lung cancer survivorship: risk of second malignancies and need for individualized care plan. J Thorac Oncol. 2012;7:1252–6.
Hamaji M, Allen MS, Cassivi SD, Deschamps C, Nichols FC, Wigle DA, et al. Surgical treatment of metachronous second primary lung cancer after complete resection of non-small cell lung cancer. J Thorac Cardiovasc Surg. 2013;145:683–90.
Hori M, Matsuda T, Shibata A, Katanoda K, Sobue T, Nishimoto H, Japan Cancer Surveillance Research Group. Cancer incidence and incidence rates in Japan in 2009: a study of 32 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 2015;45:884–91.
Acknowledgements
This study was supported by a grant-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (#25462174).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Sonobe, M., Hamaji, M., Motoyama, H. et al. Adjuvant vinorelbine and cisplatin after complete resection of stage II and III non-small cell lung cancer: long-term follow-up of our study of Japanese patients. Surg Today 48, 687–694 (2018). https://doi.org/10.1007/s00595-018-1646-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-018-1646-7