Abstract
Purpose
Despite the efficacy of postoperative adjuvant cisplatin (CDDP)-based chemotherapy for patients who have undergone surgical resection of non-small cell lung cancer (NSCLC), few reports have presented survival data for Asian patients treated with adjuvant chemotherapy involving a combination of CDDP and vinorelbine (VNR). This study was performed to evaluate the survival of patients with NSCLC who received postoperative adjuvant chemotherapy comprising CDDP + VNR.
Methods
We retrospectively evaluated patients with NSCLC who received adjuvant chemotherapy comprising CDDP + VNR at the Shizuoka Cancer Center between February 2006 and October 2011.
Results
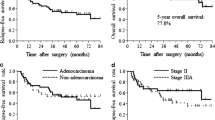
One hundred patients who underwent surgical resection of NSCLC were included in this study. The patients’ characteristics were as follows: median age 63 years (range 36–74 years), female 34%, never-smokers 20%, and non-squamous NSCLC 73%. Pathological stages IIA, IIB, and IIIA were observed in 31, 22, and 47% of patients, respectively. The 5- and 2-year overall survival rates were 73 and 93%, respectively. The 5- and 2-year relapse-free survival rates were 53 and 62%, respectively. Univariate analysis of prognostic factors showed that patient characteristics (sex, histology, and pathological stage) and CDDP dose intensity were not significantly associated with survival. In 48 patients who developed NSCLC recurrence, the 5-year survival rate after recurrence was 29%, and the median survival time after recurrence was 37 months.
Conclusions
Our results suggest that the prognosis after surgical resection of NSCLC and adjuvant chemotherapy comprising CDDP + VNR might be improving compared with previous survival data of adjuvant chemotherapy for NSCLC.




Similar content being viewed by others
References
Asamura H, Goya T, Koshiishi Y, Sohara Y, Eguchi K, Mori M, Nakanishi Y, Tsuchiya R, Shimokata K, Inoue H, Nukiwa T, Miyaoka E (2008) A Japanese lung cancer registry study: prognosis of 13,010 resected lung cancers. J Thorac Oncol 3(1):46–52. doi:10.1097/JTO.0b013e31815e8577
Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J (2004) Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 350(4):351–360. doi:10.1056/NEJMoa031644
Winton T, Livingston R, Johnson D, Rigas J, Johnston M, Butts C, Cormier Y, Goss G, Inculet R, Vallieres E, Fry W, Bethune D, Ayoub J, Ding K, Seymour L, Graham B, Tsao MS, Gandara D, Kesler K, Demmy T, Shepherd F (2005) Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med 352(25):2589–2597. doi:10.1056/NEJMoa043623
Douillard JY, Rosell R, De Lena M, Carpagnano F, Ramlau R, Gonzales-Larriba JL, Grodzki T, Pereira JR, Le Groumellec A, Lorusso V, Clary C, Torres AJ, Dahabreh J, Souquet PJ, Astudillo J, Fournel P, Artal-Cortes A, Jassem J, Koubkova L, His P, Riggi M, Hurteloup P (2006) Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB–IIIA non-small-cell lung cancer (adjuvant navelbine international trialist association [ANITA]): a randomised controlled trial. Lancet Oncol 7(9):719–727. doi:10.1016/S1470-2045(06)70804-X
Pignon JP, Tribodet H, Scagliotti GV, Douillard JY, Shepherd FA, Stephens RJ, Dunant A, Torri V, Rosell R, Seymour L, Spiro SG, Rolland E, Fossati R, Aubert D, Ding K, Waller D, Le Chevalier T (2008) Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE collaborative group. J Clin Oncol 26(21):3552–3559. doi:10.1200/JCO.2007.13.9030
Kenmotsu H, Ohde Y, Shukuya T, Eida H, Akamatsu H, Ono A, Nakamura Y, Tsuya A, Kaira K, Naito T, Murakami H, Takahashi T, Maniwa T, Isaka M, Endo M, Kondo H, Yamamoto N (2012) Feasibility of postoperative adjuvant chemotherapy of cisplatin plus vinorelbine for completely resected non-small-cell lung cancer: a retrospective study in Japan. Respir Investig 50(4):157–161. doi:10.1016/j.resinv.2012.09.002
Sawabata N, Miyaoka E, Asamura H, Nakanishi Y, Eguchi K, Mori M, Nomori H, Fujii Y, Okumura M, Yokoi K (2011) Japanese lung cancer registry study of 11,663 surgical cases in 2004: demographic and prognosis changes over decade. J Thorac Oncol 6(7):1229–1235. doi:10.1097/JTO.0b013e318219aae2
Iwamoto Y, Mitsudomi T, Sakai K, Yamanaka T, Yoshioka H, Takahama M, Yoshimura M, Yoshino I, Takeda M, Sugawara S, Kawaguchi T, Takahashi T, Ohta M, Ichinose Y, Atagi S, Okada M, Saka H, Nakagawa K, Nakanishi Y, Nishio K (2015) Randomized phase II study of adjuvant chemotherapy with long-term S-1 versus cisplatin+ S-1 in completely resected stage II–IIIA non-small cell lung cancer. Clin Cancer Res 21(23):5245–5252. doi:10.1158/1078-0432.CCR-14-3160
Sonobe M, Okubo K, Teramukai S, Yanagihara K, Sato M, Sato T, Chen F, Sato K, Fujinaga T, Shoji T, Omasa M, Sakai H, Miyahara R, Bando T, Date H (2014) Phase II study of adjuvant vinorelbine and cisplatin in Japanese patients with completely resected stage II and III non-small cell lung cancer. Cancer Chemother Pharmacol 74(6):1199–1206. doi:10.1007/s00280-014-2595-5
Feinstein AR, Sosin DM, Wells CK (1985) The Will Rogers phenomenon. Stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N Engl J Med 312(25):1604–1608. doi:10.1056/NEJM198506203122504
Liu J, Dong M, Sun X, Li W, Xing L, Yu J (2016) Prognostic value of 18F-FDG PET/CT in surgical non-small cell lung cancer: a meta-analysis. PLoS One 11(1):e0146195. doi:10.1371/journal.pone.0146195
Sekine I, Nokihara H, Yamamoto N, Kunitoh H, Ohe Y, Tamura T (2009) Comparative chemotherapeutic efficacy in non-small cell lung cancer patients with postoperative recurrence and stage IV disease. J Thorac Oncol 4(4):518–521
Ko R, Kenmotsu H, Hisamatsu Y, Akamatsu H, Omori S, Nakashima K, Oyakawa T, Wakuda K, Shukuya T, Ono A, Imai H, Taira T, Naito T, Murakami H, Mori K, Endo M, Ohde Y, Takahashi K, Takahashi T (2015) The effect of gefitinib in patients with postoperative recurrent non-small cell lung cancer harboring mutations of the epidermal growth factor receptor. Int J Clin Oncol 20(4):668–673. doi:10.1007/s10147-014-0761-8
Goss GD, O’Callaghan C, Lorimer I, Tsao MS, Masters GA, Jett J, Edelman MJ, Lilenbaum R, Choy H, Khuri F, Pisters K, Gandara D, Kernstine K, Butts C, Noble J, Hensing TA, Rowland K, Schiller J, Ding K, Shepherd FA (2013) Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol 31(27):3320–3326. doi:10.1200/JCO.2013.51.1816
Kelly K, Altorki NK, Eberhardt WE, O’Brien ME, Spigel DR, Crino L, Tsai CM, Kim JH, Cho EK, Hoffman PC, Orlov SV, Serwatowski P, Wang J, Foley MA, Horan JD, Shepherd FA (2015) Adjuvant erlotinib versus placebo in patients with stage IB–IIIA non-small-cell lung cancer (RADIANT): a randomized, double-blind, Phase III Trial. J Clin Oncol 33(34):4007–4014. doi:10.1200/JCO.2015.61.8918
Wakelee H, Dahlberg S, Keller S, Tester W, Gandara D, Graziano S, Adjei A, Leighl N, Aisner S, Rothman J, Patel J, Sborov M, Mcdermott S, Perez-Soler R, Traynor A, Butts C, Evans T, Horn L, Ramalingam S, Schiller J (2015) Randomized phase III trial of adjuvant chemotherapy with or without bevacizumab in resected non-small cell lung cancer (NSCLC): results of E1505. J Thorac Oncol 10(suppl 9):796
Acknowledgements
We thank Ms. Mie Yamada, Ms. Chiemi Asano, and Ms. Miho Watanabe (Division of Thoracic Oncology, Shizuoka Cancer Center) for their secretarial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the Japan Agency for Medical Research and Development (Grant No. 16lk0201005h0005).
Conflict of interest
Hirotsugu Kenmotsu has received grants and personal fees from AstraZeneca K.K., grants and personal fees from Chugai Pharmaceutical Co, Ltd., grants and personal fees from Boeringer Ingelheim, personal fees from Ono Pharmaceutical Co, Ltd., personal fees from Bristol-Myers K.K, grants and personal fees from Taiho Pharmaceutical Co, Ltd., personal fees from Eli Lilly K.K, personal fees from Kyowa Hakko Kirin Co., Ltd. Kazushige Wakuda has received personal fees from Eli Lilly K.K, Chugai Pharmaceutical Co, Ltd., Ono Pharmaceutical Co, Ltd., Taiho Pharmaceutical Co, Ltd., Boeringer Ingelheim. Kazuhisa Nakashima has received personal fees from Ono Pharmaceutical Co, Ltd., Taiho Pharmaceutical Co, Ltd. Shota Omori has received personal fees from AstraZeneca K.K., Chugai Pharmaceutical Co, Ltd., Ono Pharmaceutical Co, Ltd., Taiho Pharmaceutical Co, Ltd., Boeringer Ingelheim. Akira Ono has received honoraria from Chugai Pharma, Taiho Pharmaceutical and research funding to our institution from Takeda Pharmaceuticals and Taiho Pharmaceutical Co, Ltd. Tateaki Nito has received personal fees from Ono Pharmaceutical Co, Ltd. Haruyasu Murakami has received personal fees from Astrazeneca, personal fees from Chugai pharma, personal fees from Novartis, personal fees from Boehringer Ingelheim, personal fees from Phizer, personal fees from Taiho Pharmaceutical, personal fees from Lilly Japan, personal fees from Ono Pharmaceutical, personal fees from Bristol-Myers Squibb Japan, personal fees from Teijin Pharma, personal fees from Clovis Oncology, personal fees from Nippon Kayaku, other from Astellas Pharma. Masahiro Endo has received personal fees from Ono Pharmaceutical Co, Ltd. Toshiaki Takahashi has received grants from the Ministry of Health, Labour and Welfare of Japan, during the conduct of the study; grants and personal fees from AstraZeneca KK, personal fees from Boehringer Ingelheim, grants and personal fees from Pfizer Japan Inc., grants and personal fees from Eli Lilly Japan K.K., grants and personal fees from Chugai PHARMACEUTICAL CO., LTD., grants and personal fees from ONO PHARMACEUTICAL CO., LTD., grants and personal fees from Takeda Pharmaceutical Company LTD., grants from TAIHO PHARMACEUTICAL CO., LTD., grants from MSD K.K. The remaining authors declare that they have no conflict of interest.
Ethical approval
For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Kenmotsu, H., Ohde, Y., Wakuda, K. et al. Survival data for postoperative adjuvant chemotherapy comprising cisplatin plus vinorelbine after complete resection of non-small cell lung cancer. Cancer Chemother Pharmacol 80, 609–614 (2017). https://doi.org/10.1007/s00280-017-3400-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3400-z