Abstract
Aims
Glomerular damage and proximal tubular damage play an important role in the pathogenesis of diabetic kidney disease. This study aimed to investigate the relationship between the urinary markers of proximal tubular injury, including urinary liver-type fatty acid-binding protein-to-creatinine ratio (uL-FABP/Cr) and urinary N-acetyl-β-d-glucosaminidase-to-creatinine ratio (uNAG/Cr), and glycemic control status.
Methods
This cross-sectional study included 245 and 39 patients with type 2 diabetes mellitus (T2DM) and non-T2DM (NDM), respectively. The participants of this study were fitted with retrospective CGM, and glycemic control indices, such as time in range (TIR) and glycemia risk index (GRI), were calculated.
Results
The results were presented as medians (interquartile ranges). The uL-FABP/Cr was significantly higher in the microalbuminuria than in the normo-albuminuria group [4.2 (2.7–7.1) and 2.2 (1.4–3.4) μg/gCr, respectively, P < 0.001], while the uNAG/Cr in the normo-albuminuria group [6.3 (4.5–10.1) U/gCr] was significantly higher than that in the NDM group [5.3 (3.8–6.3) U/gCr, P = 0.048] but significantly lower than that in the microalbuminuria group [9.2 (6.4–11.1) U/gCr, P = 0.004]. The multivariate logistic regression analysis indicated that CGM-derived TIR was significantly associated with the urinary albumin-to-creatinine ratio [uAlb/Cr, odds ratio (OR) 0.985, 95% confidence interval (CI) 0.971–0.998, P = 0.029] and uNAG/Cr (OR 0.973, 95% CI 0.957–0.989, P = 0.001) independent of renal function. GRI was similarly associated with uAlb/Cr and uNAG/Cr.
Conclusion
The findings of this study indicated that uNAG/Cr was elevated before albuminuria development and was associated with CGM-derived TIR and GRI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic nephropathy (DN) is one of the microvascular complications of diabetes mellitus (DM) and a major cause of end-stage renal failure [1,2,3,4]. The number of patients with DM is increasing worldwide, and the prevention of DM onset and progression has become critical clinical issues [1,2,3,4]. The appearance of microalbuminuria precedes the decline in the estimated glomerular filtration rate (eGFR) in typical DN [3]. Microalbuminuria is associated with the loss of glomerular charge selectivity, and the urinary albumin-to-creatinine ratio (uAlb/Cr) is used as an early marker of DN and glomerular hyperfiltration [4, 5]. Therefore, the uAlb/Cr and eGFR have been used as measures to evaluate DN in numerous clinical trials [4]. However, many patients with DM and renal dysfunction did not have albuminuria [2, 3, 6]. Against this background, the disease concept for DN has changed, and the term diabetic kidney disease (DKD) is now widely used [7].
Many reports have suggested that in addition to glomerular hyperfiltration and glomerular hemodynamic disturbances, proximal tubular damage plays an important role in the pathogenesis of DKD [8,9,10]. Urinary markers of tubular injury include urinary liver-type fatty acid-binding protein-to-creatinine ratio (uL-FABP/Cr) and urinary N-acetyl-β-d-glucosaminidase-to-creatinine ratio (uNAG/Cr) [11]. These urinary biomarkers have been reported to be useful for the early diagnosis of DKD onset, and their excretion rate increases with DKD progression [11,12,13,14,15,16,17,18].
Previous studies have reported that glycemic variability indices and time in range (TIR) assessed by continuous glucose monitoring (CGM) are associated with uAlb/Cr [19, 20]. However, it has been reported that uAlb/Cr is not elevated in many DKD cases, and urinary tubular injury markers are useful in predicting DKD onset [8,9,10]. Owing to the association between longer TIRs and a reduced risk of diabetes complications [21, 22] and their superior accuracy in assessing hypoglycemic and hyperglycemic status compared with the traditional measure, HbA1c [23, 24], recent international consensus has underscored the significance of TIR in DM management [25]. Nonetheless, few studies have investigated the association between CGM-derived glycemic control indices and markers of tubular damage. Therefore, this study aimed to investigate the association between CGM-derived glycemic control indices such as TIR and GRI and uAlb/Cr used as a marker of glomerular damage, and uL-FABP/Cr and uNAG/Cr used as markers of tubular injury in patients with type 2 diabetes mellitus (T2DM) and non-diabetes mellitus (NDM) cases.
Methods
Study design and subjects
The present study was conducted as part of the Hyogo Diabetes Hypoglycemia Cognition Complications (HDHCC) study. The HDHCC study is a multicenter cohort study that investigated the relationship between glycemic control status and chronic vascular complications in patients with at least one cardiovascular risk factor, including diabetes, hypertension, dyslipidemia, and obesity, who visited outpatient clinics. This study included T2DM and NDM subjects aged 40 and 80 years who completed CGM and urinalysis at Hyogo Medical University Hospital between April 2018 and January 2023. The diagnosis of T2DM was made based on the guidelines of the Japan Diabetes Society [26]. In short, the diabetologists diagnosed T2DM for individuals with a fasting plasma glucose of ≥ 126 mg/dL, causal plasma glucose of ≥ 200 mg/dL, 2-h plasma glucose of ≥ 200 mg/dL during a 75-g oral glucose tolerance test, or HbA1c of ≥ 6.5% and those who had been diagnosed with T2DM or those who were on diabetes medications [26]. Moreover, the diabetologists diagnosed NDM for participants in the HDHCC study who did not meet any of the aforementioned diagnostic criteria for T2DM [26].
The exclusion criteria were as follows: (i) those with type 1 DM, (ii) those with nephrotic syndrome (proteinuria of ≥ 3.5 g/gCr at spot urine) or chronic renal failure (eGFR of < 30 mL/min/1.73 m2) [27], (iii) those with severe hepatic dysfunction (defined as alanine transaminase ≥ 3 times the upper limit of normal), (iv) those in whom CGM data could not be obtained for >7 consecutive days, (v) those who underwent renal transplantation, and (vi) those deemed ineligible for this study by their physician.
Evaluation of DKD
A urinalysis was performed in the morning on the same day as the CGM was worn. Urinary albumin was measured with an immunoturbidimetric method using Autowaco Microalbumin (Fujifilm Wako Pure Chemicals Corp., Osaka, Japan). Urinary creatinine was measured with an enzymatic method using Cygnus Auto CRE (Sino-Test Corp., Tokyo, Japan). In addition, uL-FABP was measured with the chemiluminescent enzyme immunoassay method using Lumipulse Presto L-FABP (Fujirebio Co., Ltd., Tokyo, Japan), and uNAG was measured with the colorimetric method using L-Type Wako NAG (FUJIFILM Wako Pure Chemical Corp., Osaka, Japan). The lower limit of measurement was 3 μg/mL for urinary albumin, 0.5 ng/mL for uL-FABP, and 0.4 U/L for uNAG. If the measured value was less than the lower limit of measurement, the lower limit of measurement/Cr was substituted.
CGM
Participants were instructed to wear FreeStyle Libre Pro® (Abbott Japan, Tokyo, Japan) as a CGM for 14 days. All sensor glucose data from the day after CGM attachment were analyzed according to the consensus statement of CGM and metrics for clinical trials [25]. Then, TIR (percentage of time spent in the consensus target glucose range of 70–180 mg/dL), time in the tight range (percentage of time spent in the target glucose range 70–140 mg/dL, TIT), time above range (percentage of time spent with > 180 mg/dL, TAR), time below range (percentage of time spent with < 70 mg/dL, TBR), coefficient of variation (CV), standard deviation (SD) of mean glucose, and glucose management indicator (GMI) were calculated according to the consensus report [25]. The glycemia risk index (GRI) was calculated using the following formula based on a previous report [28].
GRI = (3.0 × TBR<54 mg/dL) + (2.4 × TBR54−69 mg/dL) + (1.6 × TAR>250 mg/dL) + (0.8 × TAR181−250 mg/dL)
(Note that the upper limit of GRI was set at 100.)
Other parameters
Blood samples were taken at the time of wearing the CGM device. HbA1c was measured with high-performance liquid chromatography using HLC-723G11 (Tosoh Corporation, Tokyo, Japan). Serum creatinine levels were measured with an enzymatic method using Cygnus Auto CRE (Sino-Test Corp., Tokyo, Japan), and serum cystatin C levels were measured with the gold colloid agglutination method using Nescort GC Cystatin C (Nm) (Alfresa Pharma Corp., Osaka, Japan). The eGFR and cystatin C with eGFR (eGFRcys) for each participant were calculated from the serum creatinine and serum cystatin C levels based on previous reports [29, 30].
We defined dyslipidemia as a low-density lipoprotein cholesterol level of ≥ 140 mg/dL, high-density lipoprotein cholesterol level of < 40 mg/dL, and triglyceride level of ≥ 150 mg/dL or receiving treatment for dyslipidemia. Hypertension was defined as a systolic blood pressure of ≥ 140 mmHg, diastolic blood pressure of ≥ 90 mmHg, or receiving treatment for hypertension.
Statistical analysis
The results were presented as medians (interquartile ranges) unless otherwise stated. Patients with T2DM were divided into three groups based on uAlb/Cr values: normo-albuminuria (< 30.0 mg/gCr, normo), microalbuminuria (30.0–299.9 mg/gCr, micro), and macroalbuminuria (≥ 300.0 mg/gCr, macro) groups. Then, the normo group was used as a control and compared with the micro, macro, and NDM groups. Then, patients with T2DM were divided into two groups: TIR < 70.0% (low-TIR group) and TIR ≥ 70.0% (high-TIR group). The high-TIR group was used as a control and compared with the low-TIR and NDM groups. The Kruskal–Wallis and Steel tests were used to determine differences in quantitative data, and the chi-squared test was used to find differences in qualitative data between the groups.
The ordinal logistic regression analysis investigated the association between albuminuria categories and each glycemic control index. In Model 1, ordinal logistic regression analysis was performed with stages of albuminuria as the objective variable and each glycemic control index as explanatory variables, adjusted for duration of diabetes, sex, age, body mass index (BMI), smoking status, and presence of hypertension and dyslipidemia. Model 2 was adjusted for eGFR in addition to the factors in Model 1. Based on previous reports, a uL-FABP/Cr of > 8.4 μg/gCr as uL-FABP/Cr positive and a uNAG/Cr of > 5.8 U/gCr as uNAG/Cr positive [12, 31]. Binary logistic regression analysis was performed for tubular injury markers as objective variables and each glycemic control index as explanatory variables. Model 1 was adjusted for duration of diabetes, sex, age, BMI, smoking status, and presence of hypertension and dyslipidemia. Model 2 was adjusted for eGFR and uAlb/Cr in addition to the factors in model 1.
Statistical analyses were conducted using the BellCurve software version 4.05 (Social Survey Research Information Co., Ltd., Tokyo, Japan), with P < 0.05 indicating statistical significance.
Results
Participant background
The characteristics of the participants are shown in Table 1. The study enrolled 284 participants, consisting of 245 patients with T2DM and 39 cases with NDM. As comorbidities, 69.0% and 80.0% of the participants with T2DM had hypertension and dyslipidemia, respectively. In the NDM group, 74.4% had hypertension, while 79.5% had dyslipidemia. The NDM group was significantly older than the normo group [71 (68–74) vs. 68 (62–72) years old, P = 0.010]. In addition, the NDM group had significantly lower HbA1c levels but significantly higher TIR compared with the normo group (all P < 0.001).
Differences in renal function and urinary biomarkers based on DKD stages
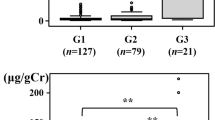
The results of eGFR, eGFRcys, and urinary biomarkers in each group are shown in Fig. 1. The eGFR was not significantly different between the normo group and the micro group [73.0 (65.0–82.0) vs. 72.5 (64.0–92.8) mL/min/1.73 m2, P = 0.940]; however, the rates in the macro group [60.0 (51.0–68.0) mL/min/1.73 m2, P = 0.001] and NDM group [65.0 (57.5–74.5) mL/min/1.73 m2, P = 0.026] were significantly lower than those in the normo group. For eGFRcys, only the macro group had significantly lower eGFRcys than the normo group did [56.9 (41.2–66.2) vs. 78.3 (67.5–87.8) mL/min/1.73 m2, P < 0.001].
Relationship between the urinary albumin excretion rate and renal function and urinary biomarkers. A Estimated glomerular filtration rate (eGFR), B cystatin C with eGFR (eGFRcys), C urinary albumin-to-creatinine ratio (uAlb/Cr), D urinary liver-type fatty acid-binding protein-to-creatinine ratio (uL-FABP/Cr), and E urinary N-acetyl-β-d-glucosaminidase-to-creatinine ratio (uNAG/Cr). Data are shown in box and whisker plots. The black dots are outliers. Macro, macroalbuminuria; Micro, microalbuminuria; NDM, non-diabetes mellitus; Normo: normo-albuminuria; T2DM, type 2 diabetes mellitus
The uAlb/Cr in the normo group was not significantly different from that in the NDM group [10.0 (6.1–15.4) vs. 10.1 (6.4–17.7) mg/gCr, P = 0.939] but increased significantly with a worsening DKD stage. The uL-FABP/Cr was not significantly different between the normo group and the NDM group [2.2 (1.4–3.4) vs. 2.2 (1.4–3.6) μg/gCr, P = 0.990], whereas the micro group [4.2 (2.7–7.1) μg/gCr, P < 0.001] and the macro group [15.4 (10.0–26.6) μg/gCr, P < 0.001] had significantly higher uL-FABP/Cr than the normo group. The uNAG/Cr was significantly lower in the NDM group [5.3 (3.8–6.3) U/gCr, P = 0.048] but was significantly higher in the micro [9.2 (6.4–11.1) U/gCr, P = 0.004] and macro [13.6 (9.5–19.3) U/gCr, P < 0.001] groups than in the normo group [6.3 (4.5–10.1) U/gCr].
Differences in renal function and urinary biomarkers based on TIR
Patients with T2DM were divided into two groups based on TIR, and renal function and urinary biomarkers in the low-TIR and NDM groups were compared with those in the high-TIR group as controls (Table 2 and Fig. 2). No significant difference in eGFRcys was found between the low-TIR [72.6 (55.9–84.2) mL/min/1.73 m2, P = 0.063] and NDM [72.0 (61.9–80.6) mL/min/1.73 m2, P = 0.191] groups and the high-TIR group [75.6 (66.3–87.4) mL/min/1.73 m2]. The uAlb/Cr was significantly higher in the low-TIR group than in the high-TIR group [21.2 (8.8–90.7) vs. 13.9 (6.8–34.6) mg/gCr, P = 0.026], whereas the NDM group [10.1 (6.4–17.7) mg/gCr, P = 0.099] did not show significant difference from the high-TIR group. Similar results were obtained for the uL-FABP/Cr. The uNAG/Cr was significantly lower in the NDM group than in the high-TIR group [5.3 (3.8–6.3) vs. 6.7 (4.5–10.4) U/gCr, P = 0.009], whereas it was significantly higher in the low-TIR group [9.0 (5.8–12.3) U/gCr, P = 0.006] than in the high-TIR group.
Relationship between the time in range (TIR) and renal function and urinary biomarkers. A Estimated glomerular filtration rate (eGFR), B cystatin C with eGFR (eGFRcys), C urinary albumin-to-creatinine ratio (uAlb/Cr), D urinary liver-type fatty acid-binding protein-to-creatinine ratio (uL-FABP/Cr), and E urinary N-acetyl-β-d-glucosaminidase-to-creatinine ratio (uNAG/Cr). Data are shown in box and whisker plots. The black dots are outliers. Macro, macroalbuminuria; Micro, microalbuminuria; Normo, normo-albuminuria; NDM, non-diabetes mellitus; T2DM, type 2 diabetes mellitus
Relationship between urinary biomarkers and each glycemic control index
The relationship between the CGM-derived glycemic control indices and uAlb/Cr is shown in Table 3. In the univariate analysis, the uAlb/Cr was significantly associated with TIR (crude odds ratio (OR) 0.980, 95% confidence interval (CI) 0.967–0.992, P = 0.002), TIT (crude OR 0.981, 95% CI 0.970–0.992, P < 0.001), TAR (crude OR 1.021, 95% CI 1.009–1.034, P < 0.001), and SD (crude OR 1.023, 95% CI 1.003–1.042, P = 0.020). In addition, a significant association was found between the uAlb/Cr and GRI, an indicator of risk for hyperglycemia and hypoglycemia (crude OR 1.013, 95% CI 1.003–1.024, P = 0.012). On the contrary, no significant association was found between the uAlb/Cr and TBR (crude OR 0.974, 95% CI 0.929–1.021, P = 0.275) and CV (crude OR 0.998, 95% CI 0.960–1.036, P = 0.907). In Model 2, which was adjusted for multiple factors, such as eGFR, duration of diabetes, sex, age, BMI, smoking status, and presence of hypertension and dyslipidemia, a significant association was found between uAlb/Cr and each of the CGM-derived glycemic control indices, such as TIR (OR 0.985, 95% CI 0.971–0.998, P = 0.029), TAR (OR 1.015, 95% CI 1.002–1.029, P = 0.028), and GRI (OR 1.012, 95% CI 1.001–1.024, P = 0.038). Although GMI (OR 1.485, 95% CI 1.067–2.067, P = 0.019) was significantly associated with uAlb/Cr, no significant association was observed between HbA1c (OR 1.127, 95% CI 0.807–1.574, P = 0.483) and uAlb/Cr.
The relationship between the uL-FABP/Cr and uNAG/Cr and each glycemic control index is shown in Table 4. In the univariate analysis, the uL-FABP/Cr was significantly associated with TIR (crude OR 0.977, 95% CI 0.961–0.993, P = 0.004), TIT (crude OR 0.982, 95% CI 0.967–0.996, P = 0.014), TAR (crude OR 1.022, 95% CI 1.006–1.038, P = 0.006), and GRI (Crude OR 1.018, 95% CI 1.004–1.031, P = 0.010). Similar results were obtained in Model 1, which was adjusted for multivariate factors. However, the logistic regression analysis adjusted for uAlb/Cr and eGFR showed no significant association between uL-FABP/Cr and each CGM-derived glycemic control index. On the contrary, the multivariate analysis indicated that uNAG/Cr was significantly associated not only with TIR (OR 0.973, 95% CI 0.957–0.989, P = 0.001) and TIT (OR 0.981, 95% CI 0.968–0.993, P = 0.003) but also with TAR (OR 1.024, 95% CI 1.008–1.040, P = 0.004) and GRI (OR 1.019, 95% CI 1.006–1.032, P = 0.004).
Discussion
The findings of this study indicated that uL-FABP/Cr is significantly elevated in the early stages of DKD with microalbuminuria in patients with T2DM. On the contrary, uNAG/Cr was significantly higher even in patients with T2DM and normo-albuminuria than in those with NDM, and uNAG/Cr increased with worsening DKD stage. Furthermore, not only uAlb/Cr but also uNAG/Cr were significantly associated with CGM-derived glycemic control indices, such as TIR, TIT, and GRI, independent of renal function and the presence of hypertension.
Consistent with previous reports, the results of this study indicated that uAlb/Cr, used as a marker of glomerular damage, was associated with CGM-derived TIR and GRI [19, 20, 32, 33]. As both TIR and GRI are considered indicators of the quality of glycemic control [28], glomerular dysfunction may be associated with poorer quality of glycemic control. On the other hand, several studies have suggested that proximal tubular damage is important in the DKD onset and progression and that tubular damage precedes glomerular damage [8,9,10]. Several renal proximal tubular injury markers have been detected in the urine of patients with T2DM but without overt glomerular damage. For example, uL-FABP/Cr and uNAG/Cr have been reported to be significantly higher in T2DM with normo-albuminuria than in NDM [11,12,13,14,15,16,17,18]. The results of this study showed that the normo-albuminuria group had a significantly higher uNAG/Cr than the NDM group, whereas uL-FABP/Cr did not differ significantly between the two groups. The relationship between increased uL-FABP/Cr and DKD severity varies between studies [11,12,13, 34]; however, uNAG/Cr precedes increased urinary albumin excretion [14,15,16,17,18, 35].
UL-FABP/Cr and uNAG/Cr are urinary biomarkers of tubular damage. L-FABP is located in the cytoplasm of human renal proximal tubular cells and is engaged in free fatty acid metabolism [34]. UL-FABP/Cr increases with excessive stress on the tubules, including urinary protein excretion, hypertension, tubular ischemia, and oxidative stress [36,37,38]. In addition, uL-FABP/Cr decreases along with uAlb/Cr using RAS inhibitors or statins [37,38,39]. This study showed no significant association between uL-FABP/Cr and CGM-derived glycemic control indices in a multivariate analysis adjusted for uAlb/Cr and eGFR. Thus, uL-FABP/Cr may be related to factors, such as the presence of albuminuria or eGFR, rather than glycemic control in patients with T2DM.
In contrast to uL-FABP/Cr, this study showed that uNAG/Cr was associated with CGM-derived glycemic control indices, such as TIR and GRI, independent of the uAlb/Cr and eGFR levels. NAG is one of the glycoproteinases contained in intracellular lysosomes, and its urinary excretion is increased in response to the injury of lysosomes in tubular epithelial cells [11]. Zheng HJ et al. reported that sustained hyperglycemic exposure may impair lysosomal function [40]. Furthermore, a positive correlation was found between uNAG/Cr and blood glucose levels and uNAG/Cr increases with high glucose load [41,42,43]. Thus, among the urinary biomarkers, uNAG/Cr is assumed to be more sensitive to hyperglycemia [44, 45]. In fact, the findings of this study indicated that TAR and GRI were associated with uNAG/Cr. In addition, the results also showed that TIT was associated with uNAG/Cr. Because uNAG/Cr decreases with lowering blood glucose levels [46, 47], increasing the percentage of time spent in the 70–140 mg/dL range might decrease uNAG/Cr. Although further validation is needed to elucidate the pathogenesis mechanism described above, this study is valuable as the first to show an association between newly developed glycemic control indices such as GRI and TIR and tubular damage markers in patients with DKD.
This study had some limitations. First, it was a cross-sectional study. Therefore, long-term prospective studies on the relationship between urinary biomarkers and DKD progression are needed. In addition, this study measured uAlb/Cr, uL-FABP/Cr, and uNAG/Cr as urinary biomarkers. Other tubular damage markers include neutrophil gelatinase-associated lipocalin, kidney injury molecule-1, and β2-microglobulin [48]. Therefore, studies including these urinary biomarkers might be necessary.
Conclusions
The results of this study indicate that urinary NAG, which is largely located in the proximal tubule cells and is a leakage marker of epithelial injury, is significantly high even in patients with T2DM in the normo-albuminuria stage. Furthermore, this study showed for the first time that uNAG/Cr was associated with the CGM-derived glycemic control indices, such as TIR and GRI, independent of albuminuria and eGFR levels.
Data availability
The individual deidentified participant data will be shared upon reasonable request to the corresponding author.
Abbreviations
- DN:
-
Diabetic nephropathy
- DM:
-
Diabetes mellitus
- eGFR:
-
Estimated glomerular filtration rate
- uAlb/Cr:
-
Urinary albumin-to-creatinine ratio
- DKD:
-
Diabetic kidney disease
- uL-FABP/Cr:
-
Urinary liver-type fatty acid-binding protein-to-creatinine ratio
- uNAG/Cr:
-
Urinary N-acetyl-β-d-glucosaminidase-to-creatinine ratio
- TIR:
-
Time in range
- CGM:
-
Continuous glucose monitoring
- T2DM:
-
Type 2 diabetes mellitus
- NDM:
-
Non-diabetes mellitus
- HDHCC:
-
Hyogo Diabetes Hypoglycemia Cognition Complications
- TIT:
-
Time in the tight range
- TAR:
-
Time above range
- TBR:
-
Time below range
- CV:
-
Coefficient of variation
- SD:
-
Standard deviation
- GMI:
-
Glucose management indicator
- GRI:
-
Glycemia risk index
- eGFRcys:
-
Cystatin C with eGFR
- OR:
-
Odds ratio
- CI:
-
Confidence interval
References
Levin A, Tonelli M, Bonventre et al (2017) Global kidney health and beyond: a roadmap for closing gaps in care, research, and policy. Lancet 390(10105):1888–1917
Tuttle KR, Agarwal R, Alpers CE et al (2022) Molecular mechanisms and therapeutic targets for diabetic kidney disease. Kidney Int 102(2):248–260
Pugliese G, Penno G, Natali A et al; Italian Diabetes Society and the Italian Society of Nephrology (2019) Diabetic kidney disease: new clinical and therapeutic issues. Joint position statement of the Italian Diabetes Society and the Italian Society of Nephrology on “The natural history of diabetic kidney disease and treatment of hyperglycemia in patients with type 2 diabetes and impaired renal function”. Nutr Metab Cardiovasc Dis 29(11):1127–1150
ElSayed NA, Aleppo G, Aroda VR et al (2023) 11 Chronic Kidney Disease and Risk Management: standards of care in diabetes—2023. Diabetes Care 46(Suppl 1):S191–S202
Singh A, Satchell SC (2011) Microalbuminuria: causes and implications. Pediatr Nephrol 26(11):1957–1965
Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR (2006) UKPDS study group risk factors for renal dysfunction in type 2 diabetes: UK prospective diabetes study 74. Diabetes 55(6):1832–1839
Selby NM, Taal MW (2020) An updated overview of diabetic nephropathy: diagnosis, prognosis, treatment goals and latest guidelines. Diabetes Obes Metab 22(Suppl 1):3–15
Gilbert RE (2017) Proximal tubulopathy: prime mover and key therapeutic target in diabetic kidney disease. Diabetes 66(4):791–800
Zeni L, Norden AG, Cancarini G, Unwin RJ (2017) A more tubulocentric view of diabetic kidney disease. J Nephrol 30(6):701–717
Thethi TK, Batuman V (2019) Challenging the conventional wisdom on diabetic nephropathy: Is microalbuminuria the earliest event? J Diabetes Complicat 33(3):191–192
Liu H, Feng J, Tang L. Early renal structural changes and potential biomarkers in diabetic nephropathy. Fron Physiol 2022:2364
Kamijo-Ikemori A, Sugaya T, Yasuda T et al (2011) Clinical significance of urinary liver-type fatty acid–binding protein in diabetic nephropathy of type 2 diabetic patients. Diabetes Care 34(3):691–696
Thi TND, Gia BN, Le Thi HL, Thi TNC, Thanh HP (2020) Evaluation of urinary L-FABP as an early marker for diabetic nephropathy in type 2 diabetic patients. J Med Biochem 39(2):224–230
Mohammadi-Karakani A, Asgharzadeh-Haghighi S, Ghazi-Khansari M, Hosseini R (2007) Determination of urinary enzymes as a marker of early renal damage in diabetic patients. J Clin Lab Anal 21(6):413–417
Nauta FL, Boertien WE, Bakker SJ et al (2011) Glomerular and tubular damage markers are elevated in patients with diabetes. Diabetes Care 34(4):975–981
Vaidya VS, Niewczas MA, Ficociello LH et al (2011) Regression of microalbuminuria in type 1 diabetes is associated with lower levels of urinary tubular injury biomarkers, kidney injury molecule-1, and N-acetyl-β-d-glucosaminidase. Kidney Int 79(4):464–470
Sheira G, Noreldin N, Tamer A, Saad M (2015) Urinary biomarker N-acetyl-β-d-glucosaminidase can predict severity of renal damage in diabetic nephropathy. J Diabetes Metab Disord 14:4
Assal HS, Tawfeek S, Rasheed EA, El-Lebedy D, Thabet EH (2013) Serum cystatin C and tubular urinary enzymes as biomarkers of renal dysfunction in type 2 diabetes mellitus. Clin Med Insights Endocrinol Diabetes 6:7–13
Yoo JH, Choi MS, Ahn J et al (2020) Association between continuous glucose monitoring-derived time in range, other core metrics, and albuminuria in type 2 diabetes. Diabetes Technol Ther 22(10):768–776
Jin X, Yang X, Xu Y et al (2023) Differential correlation between time in range and eGFR or albuminuria in type 2 diabetes. Diabetol Metab Syndr 15(1):92
Lu J, Ma X, Zhou J et al (2018) Association of time in range, as assessed by continuous glucose monitoring, with diabetic retinopathy in type 2 diabetes. Diabetes Care 41(11):2370–2376
Beck RW, Bergenstal RM, Riddlesworth TD et al (2019) Validation of time in range as an outcome measure for diabetes clinical trials. D iabetes Care 42(3):400–405
Danne T, Nimri R, Battelino T et al (2017) International consensus on use of continuous glucose monitoring. Diabetes Care 40(12):1631–1640
Vigersky RA, McMahon C (2019) The relationship of hemoglobin A1C to time-in-range in patients with diabetes. Diabetes Technol Ther 21(2):81–85
Battelino T, Alexander CM, Amiel SA et al (2023) Continuous glucose monitoring and metrics for clinical trials: an international consensus statement. Lancet Diabetes Endocrinol 11(1):42–57
Araki E, Goto A, Kondo T et al (2020) Japanese clinical practice guideline for diabetes 2019. J Diabetes Investig 11(4):1020–1076
Wada T, Ishimoto T, Nakaya I et al (2021) A digest of the evidence-based clinical practice guideline for nephrotic syndrome 2020. Clin Exp Nephrol 25(12):1277–1285
Klonoff DC, Wang J, Rodbard D et al (2023) A glycemia risk index (GRI) of Hypoglycemia and Hyperglycemia for continuous glucose monitoring validated by clinician ratings. J Diabetes Sci Technol 17(5):1226–1242
Matsuo S, Imai E, Horio M et al (2009) Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53(6):982–992
Tanaka M, Matsuo K, Enomoto M, Mizuno K (2004) A sol particle homogeneous immunoassay for measuring serum cystatin C. Clin Biochem 37(1):27–33
Ouchi M, Oba K, Saigusa T et al (2015) Association between pulse wave velocity and a marker of renal tubular damage (N-acetyl-β-d-glucosaminidase) in patients without diabetes. J Clin Hypertens 17(4):290–297
Yoo JH, Kim JY, Kim JH (2023) Association Between Continuous Glucose Monitoring-Derived Glycemia Risk Index and Albuminuria in Type 2 Diabetes. Diabetes Technol Ther (in press)
Kuroda N, Kusunoki Y, Osugi K et al (2021) Relationships between time in range, glycemic variability including hypoglycemia and types of diabetes therapy in Japanese patients with type 2 diabetes mellitus: hyogo diabetes hypoglycemia cognition complications study. J Diabetes Investig 12(2):244–253
Viswanathan V, Sivakumar S, Sekar V, Umapathy D, Kumpatla S (2015) Clinical significance of urinary liver-type fatty acid binding protein at various stages of nephropathy. Indian J Nephrol 25(5):269–273
Bouvet BR, Paparella CV, Arriaga SM, Monje AL, Amarilla AM, Almará AM (2014) Evaluation of urinary N-acetyl-beta-D-glucosaminidase as a marker of early renal damage in patients with type 2 diabetes mellitus. Arq Bras Endocrinol Metabol 58(8):798–801
Kamijo A, Sugaya T, Hikawa A et al (2004) Urinary excretion of fatty acid-binding protein reflects stress overload on the proximal tubules. Am J Pathol 165(4):1243–1255
Kamijo-Ikemori A, Sugaya T, Ichikawa D et al (2013) Urinary liver type fatty acid binding protein in diabetic nephropathy. Clin Chim Acta 424:104–108
Yamamoto T, Noiri E, Ono Y et al (2007) Renal L-type fatty acid–binding protein in acute ischemic injury. J Am Soc Nephrol 18(11):2894–2902
Nielsen SE, Sugaya T, Tarnow L et al (2009) Tubular and glomerular injury in diabetes and the impact of ACE inhibition. Diabetes Care 32(9):1684–1688
Zheng HJ, Zhang X, Guo J et al (2020) Lysosomal dysfunction–induced autophagic stress in diabetic kidney disease. J Cell Mol Med 24(15):8276–8290
Kim SR, Lee Y-H, Lee S-G et al (2016) Urinary N-acetyl-β-d-glucosaminidase, an early marker of diabetic kidney disease, might reflect glucose excursion in patients with type 2 diabetes. Medicine 95(27):e4114
Ouchi M, Suzuki T, Hashimoto M et al (2012) Urinary N-acetyl-β-d-glucosaminidase levels are positively correlated with 2-hr plasma glucose levels during oral glucose tolerance testing in prediabetes. J Clin Lab Anal 26(6):473–480
Ishii N, Ikenaga H, Ogawa Z, Aoki Y, Saruta T, Suga T (2001) Effects of renal sorbitol accumulation on urinary excretion of enzymes in hyperglycaemic rats. Ann Clin Biochem 38(Pt 4):391–398
Han E, Kim M-K, Lee Y-H, Kim HS, Lee B-W (2019) Association between nonalbumin proteinuria and renal tubular damage of N-acetyl-β-d-glucosaminidase and its clinical relevance in patients with type 2 diabetes without albuminuria. J Diabetes Complicat 33(3):255–260
Watanabe Y, Nunoi K, Maki Y, Nakamura Y, Fujishima M (1987) Contribution of glycemic control to the levels of urinary N-acetyl-beta-D-glucosaminidase (NAG), total protein, beta 2-microglobulin and serum NAG in type 1 (insulin-dependent) diabetes mellitus without macroalbuminuria. Clin Nephrol 28(5):227–231
UKPDS Study Group (1993) UK Prospective Diabetes Study (UKPDS). IX: relationships of urinary albumin and N-acetylglucosaminidase to glycaemia and hypertension at diagnosis of Type 2 (non-insulin-dependent) diabetes mellitus and after 3 months diet therapy. Diabetologia 36(9):835–42
Brouhard BH, LaGrone L, Travis LB, Pollard TG (1984) Response of urinary N-acetyl-beta-D-glucosaminidase to rapid decreases in blood glucose. Clin Chim Acta 140(2):197–202
Rotbain Curovic V, Hansen TW, Eickhoff MK et al (2018) Urinary tubular biomarkers as predictors of kidney function decline, cardiovascular events and mortality in microalbuminuric type 2 diabetic patients. Acta Diabetol 55(11):1143–1150
Acknowledgements
The authors are grateful for the excellent technical assistance of MI and AM. We also wish to thank the staff of the Department of Diabetes, Endocrinology and Clinical Immunology, School of Medicine, Hyogo Medical University, as well as the participants in this study for their valuable contributions. The members of the HDHCC study group are as follows: YK, KO, MO, CI, MI, AT, CY, AM, TT, MK, AK, MK, KK, TS, TK, and HK, Hyogo Medical University; Hiroyuki Konya and Toshihiro Matsuo, Ashiya Municipal Hospital; Hideki Ifuku, Amagasaki Chuo Hospital; Daisuke Azuma, Azuma Clinic; Takeshi Fukui, Fukui Clinic; Isao Hayashi, Hayashi Clinic; Satoru Katayama, Hyogo Medical University Sasayama Medical Center; Masataka Kanyama, Masaru Usami, and Hiroki Ikeda, Ikeda Hospital; Tadahiro Inagaki, Inagaki Medical Clinic; Tomoya Hamaguchi, Itami City Hospital; Akihito Otsuka, Kawasaki Hospital; Shogo Kurebayashi, Kurebayashi Clinic; Kenji Kusunoki, Kusunoki Clinic; Minoru Kubota, Meiwa Hospital; Takeharu Sasaki, Nishinomiya Watanabe Hospital; Sachie Hirose, Satoshi Matsutani, and Shinya Makino, Osaka Gyoumeikan Hospital; Tetsuhiro Kitamura and Daisuke Tamada, Tamada Clinic; Hidenori Taniguchi, Taniguchi Medical Clinic; Nobuaki Watanabe, Watanabe Clinic; and Mitsuyoshi Namba, Takarazuka City Hospital.
Funding
This study was partly supported by the Japan Society for The Promotion of Science KAKENHI Grant Nos. 22K10541 and 21K17297. This work was also supported by “Hyogo Medical University Diversity Grant for Research Promotion” under MEXT Funds for the Development of Human Resources in Science and Technology, “Initiative for Realizing Diversity in the Research Environment (Characteristic-Compatible Type)”.
Author information
Authors and Affiliations
Consortia
Contributions
AT, YK, MO, KO, CI, and HK are responsible for designing this study, collecting the subjects, analyzing the data, and writing the manuscript. MI, TT, MK, KK, and TK are responsible for the study participants. All authors have approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of financial or non-financial interest.
Ethics approval
This study was performed in compliance with the guidelines for the Declaration of Helsinki. This study was approved by the Ethics Committee of Hyogo Medical University Hospital and the ethics review committee of each participating institution (Approval No. 4250). All participants provided informed consent and signed informed consent forms. Clinical trial registration: UMIN000032143 (https://center6.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000036617).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the topical collection Diabetic Nephropathy, Managed By Giuseppe Pugliese.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Takagi, A., Kusunoki, Y., Ohigashi, M. et al. Association between continuous glucose monitoring-derived glycemic control indices and urinary biomarkers of diabetic kidney disease: Hyogo Diabetes Hypoglycemia Cognition Complications study. Acta Diabetol 61, 413–423 (2024). https://doi.org/10.1007/s00592-023-02214-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-023-02214-9