Abstract
Background
Diabetic kidney disease (DKD) is one of the most common diabetic complications. Endoplasmic reticulum stress (ERS) is an important step for renal tubular epithelial cell apoptosis during DKD progression. Herein, the role and regulatory mechanism of METTL14 in ERS during DKD progression were investigated.
Methods
DKD animal and cell models were established by streptozotocin (STZ) and high glucose (HG), respectively. HE and Masson staining were performed to analyze renal lesions in DKD mouse. Cell viability and proliferation were determined by MTT and EdU staining, respectively. HK2 cell apoptosis was analyzed by flow cytometry. TUG1 m6A level was determined by Me-RIP. The interaction between TUG1, LIN28B and MAPK1 was analyzed by RIP and RNA pull-down assays.
Results
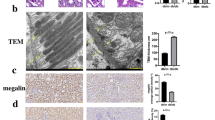
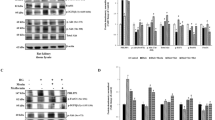
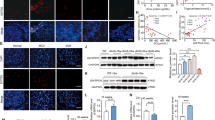
HG stimulation promoted apoptosis and increased ERS marker proteins (GRP78, CHOP and caspase12) expression in HK2 cells, while these changes were reversed by METTL14 knockdown. METTL14 inhibited TUG1 stability and expression level in an m6A-dependent manner. As expected, TUG1 knockdown abrogated METTL14 knockdown’s inhibition on HG-induced HK2 cell apoptosis and ERS. In addition, TUG1 inactivated MAPK1/ERK signaling by binding with LIN28B. And TUG1 overexpression’s repression on HG-induced HK2 cell apoptosis and ERS was abrogated by MAPK1 signaling activation. Meanwhile, METTL14 knockdown or TUG1 overexpression protected against STZ-induced renal lesions and renal fibrosis in DKD mouse.
Conclusion
METTL14 promoted renal tubular epithelial cell apoptosis and ERS by activating MAPK/ERK pathway through m6A modification of TUG1, thereby accelerating DKD progression.






Similar content being viewed by others
Availability of data and material
All data generated or analyzed during this study are included in this published article.
Abbreviations
- DM:
-
Diabetes mellitus
- DKD:
-
Diabetic kidney disease
- m6A:
-
N6 methyladenosine
- METTL14:
-
Methyltransferase methyltransferase-like 14
- LncRNAs:
-
Long noncoding RNAs
- TUG1:
-
Taurine upregulated gene 1
- STZ:
-
Streptozotocin
- HG:
-
High glucose
- MAPK:
-
Mitogen-activated protein kinases
- ERK:
-
Extracellular signal-regulation, kinase
- JNK:
-
C-Jun-N-terminal kinase
- ERS:
-
Endoplasmic reticulum stress
- RBP:
-
RNA binding protein
- MTT:
-
3-(4, 5-Dimethylthiazolyl2)-2, 5-diphenyltetrazolium bromide
- Bcl-2:
-
B-cell CLL/lymphoma 2
- GRP78:
-
Glucose-regulated protein78
- CHOP:
-
C/EBP homologous protein
- EdU:
-
Ethynyl-2′-deoxyuridine
- RIP:
-
RNA immunoprecipitation
- Me-RIP:
-
Methylated RNA binding protein immunoprecipitation
- HE:
-
Hematoxylin–eosin
- Scr:
-
Serum creatinine
- BUN:
-
Blood urea nitrogen
- qRT-PCR:
-
Quantitative real-time polymerase chain reaction
References
Maresch CC, Stute DC, Fleming T, Lin J, Hammes HP, Linn T (2019) Hyperglycemia induces spermatogenic disruption via major pathways of diabetes pathogenesis. Sci Rep 9(1):13074
VR ALBVR, Tan SH, Candasamy M, Bhattamisra SK (2019) Diabetic nephropathy: an update on pathogenesis and drug development. Diabetes Metab Syndr 13(1):754–762
Sharma D, Bhattacharya P, Kalia K, Tiwari V (2017) Diabetic nephropathy: New insights into established therapeutic paradigms and novel molecular targets. Diabetes Res Clin Pract 128:91–108
Kato M, Natarajan R (2014) Diabetic nephropathy—emerging epigenetic mechanisms. Nat Rev Nephrol 10(9):517–530
La Rocca E, Fiorina P, Astorri E, Rossetti C, Lucignani G, Fazio F et al (2000) Patient survival and cardiovascular events after kidney-pancreas transplantation: comparison with kidney transplantation alone in uremic IDDM patients. Cell Transpl 9(6):929–932
Petrazzuolo A, Sabiu G, Assi E, Maestroni A, Pastore I, Lunati ME et al (2023) Broadening horizons in mechanisms, management, and treatment of diabetic kidney disease. Pharmacol Res 190:106710
Sano R, Reed JC (2013) ER stress-induced cell death mechanisms. Biochem Biophys Acta 1833(12):3460–3470
Hu H, Tian M, Ding C, Yu S (2018) The C/EBP homologous protein (CHOP) transcription factor functions in endoplasmic reticulum stress-induced apoptosis and microbial infection. Front Immunol 9:3083
He Z, Zhou Y, Huang Y, Wang Q, Zheng B, Zhang H et al (2017) Dl-3-n-butylphthalide improves functional recovery in rats with spinal cord injury by inhibiting endoplasmic reticulum stress-induced apoptosis. Am J Transl Res 9(3):1075–1087
Zhang J, Morris MW Jr, Dorsett-Martin WA, Drake LC, Anderson CD (2013) Autophagy is involved in endoplasmic reticulum stress-induced cell death of rat hepatocytes. J Surg Res 183(2):929–935
Liu G, Sun Y, Li Z, Song T, Wang H, Zhang Y et al (2008) Apoptosis induced by endoplasmic reticulum stress involved in diabetic kidney disease. Biochem Biophys Res Commun 370(4):651–656
Roundtree IA, Evans ME, Pan T, He C (2017) Dynamic RNA modifications in gene expression regulation. Cell 169(7):1187–1200
Lei L, Bai YH, Jiang HY, He T, Li M, Wang JP (2021) A bioinformatics analysis of the contribution of m6A methylation to the occurrence of diabetes mellitus. Endocr Connect 10(10):1253–1265
Ma L, Chen T, Zhang X, Miao Y, Tian X, Yu K et al (2021) The m(6)A reader YTHDC2 inhibits lung adenocarcinoma tumorigenesis by suppressing SLC7A11-dependent antioxidant function. Redox Biol 38:101801
Lu Z, Liu H, Song N, Liang Y, Zhu J, Chen J et al (2021) METTL14 aggravates podocyte injury and glomerulopathy progression through N(6)-methyladenosine-dependent downregulating of Sirt1. Cell Death Dis 12(10):881
Li M, Deng L, Xu G (2021) METTL14 promotes glomerular endothelial cell injury and diabetic nephropathy via m6A modification of α-klotho. Mol Med 27(1):106
Schmitz SU, Grote P, Herrmann BG (2016) Mechanisms of long noncoding RNA function in development and disease. Cell Mol Life Sci 73(13):2491–2509
Zhang H, Yan Y, Hu Q, Zhang X (2021) LncRNA MALAT1/microRNA let-7f/KLF5 axis regulates podocyte injury in diabetic nephropathy. Life Sci 266:118794
Ge Y, Wang J, Wu D, Zhou Y, Qiu S, Chen J et al (2019) lncRNA NR_038323 suppresses renal fibrosis in diabetic nephropathy by targeting the miR-324-3p/DUSP1 Axis. Mol Ther Nucleic Acids 17:741–753
Wang WY, Wang YF, Ma P, Xu TP, Shu YQ (2017) Taurine-upregulated gene 1: a vital long non-coding RNA associated with cancer in humans. Mol Med Rep 16(5):6467–6471
Li SY, Susztak K (2016) The long noncoding RNA Tug1 connects metabolic changes with kidney disease in podocytes. J Clin Invest 126(11):4072–4075
Wang F, Gao X, Zhang R, Zhao P, Sun Y, Li C (2019) LncRNA TUG1 ameliorates diabetic nephropathy by inhibiting miR-21 to promote TIMP3-expression. Int J Clin Exp Pathol 12(3):717–729
Lei X, Zhang L, Li Z, Ren J (2018) Astragaloside IV/lncRNA-TUG1/TRAF5 signaling pathway participates in podocyte apoptosis of diabetic nephropathy rats. Drug Des Dev Ther 12:2785–2793
Meng L, Lin H, Huang X, Weng J, Peng F, Wu S (2022) METTL14 suppresses pyroptosis and diabetic cardiomyopathy by downregulating TINCR lncRNA. Cell Death Dis 13(1):38
Xin X, Khan ZA, Chen S, Chakrabarti S (2004) Extracellular signal-regulated kinase (ERK) in glucose-induced and endothelin-mediated fibronectin synthesis. Lab Investig 84(11):1451–1459
Sun Y, Liu WZ, Liu T, Feng X, Yang N, Zhou HF (2015) Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct Res 35(6):600–604
Liu Y, Chen J, Liang H, Cai Y, Li X, Yan L et al (2022) Human umbilical cord-derived mesenchymal stem cells not only ameliorate blood glucose but also protect vascular endothelium from diabetic damage through a paracrine mechanism mediated by MAPK/ERK signaling. Stem Cell Res Ther 13(1):258
Cheng S, Li L, Song C, Jin H, Ma S, Kang P (2020) Sitagliptin relieves diabetic nephropathy fibrosis via the MAPK/ERK signaling pathway. Minerva Endocrinol 45(3):273–275
Jin J, Ma Y, Tong X, Yang W, Dai Y, Pan Y et al (2020) Metformin inhibits testosterone-induced endoplasmic reticulum stress in ovarian granulosa cells via inactivation of p38 MAPK. Hum Reprod 35(5):1145–1158
Victor P, Umapathy D, George L, Juttada U, Ganesh GV, Amin KN et al (2021) Crosstalk between endoplasmic reticulum stress and oxidative stress in the progression of diabetic nephropathy. Cell Stress Chaperones 26(2):311–321
Xu J, Liu L, Gan L, Hu Y, Xiang P, Xing Y et al (2021) Berberine acts on C/EBPβ/lncRNA Gas5/miR-18a-5p loop to decrease the mitochondrial ROS generation in HK-2 Cells. Front Endocrinol 12:675834
Cheng Q, Pan J, Zhou ZL, Yin F, Xie HY, Chen PP et al (2021) Caspase-11/4 and gasdermin D-mediated pyroptosis contributes to podocyte injury in mouse diabetic nephropathy. Acta Pharmacol Sin 42(6):954–963
Yu XZ, Yang BW, Ao MY, Wu YK, Ye H, Wang RY et al (2023) CircNFIX stimulates the proliferation, invasion, and stemness properties of ovarian cancer cells by enhancing SH3RF3 mRNA stability via binding LIN28B. Kaohsiung J Med Sci 39(3):234–243
Tziomalos K, Athyros VG (2015) Diabetic nephropathy: new risk factors and improvements in diagnosis. Rev Diabet Stud 12(1–2):110–118
Hua F (2020) New insights into diabetes mellitus and its complications: a narrative review. Ann Transl Med 8(24):1689
Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR (2012) Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell 149(7):1635–1646
Engin F, Hotamisligil GS (2010) Restoring endoplasmic reticulum function by chemical chaperones: an emerging therapeutic approach for metabolic diseases. Diabetes Obes Metab 12(Suppl 2):108–115
Ju Y, Su Y, Chen Q, Ma K, Ji T, Wang Z et al (2019) Protective effects of astragaloside IV on endoplasmic reticulum stress-induced renal tubular epithelial cells apoptosis in type 2 diabetic nephropathy rats. Biomed Pharmacother 109:84–92
Huang S, Xu Y, Ge X, Xu B, Peng W, Jiang X et al (2019) Long noncoding RNA NEAT1 accelerates the proliferation and fibrosis in diabetic nephropathy through activating Akt/mTOR signaling pathway. J Cell Physiol 234(7):11200–11207
Zhang J, Jiang T, Liang X, Shu S, Xiang X, Zhang W et al (2019) lncRNA MALAT1 mediated high glucose-induced HK-2 cell epithelial-to-mesenchymal transition and injury. J Physiol Biochem 75(4):443–452
Pereira B, Billaud M, Almeida R (2017) RNA-binding proteins in cancer: old players and new actors. Trends Cancer 3(7):506–528
Liu Z, Bian M, Pang L (2023) LncRNA CRNDE binds hnRNPA1 to facilitate carbon monoxide poisoning-induced delayed encephalopathy via inhibiting UCHL5-mediated SMO deubiquitination. Metab Brain Dis 38(3):1097–1113
Tao W, Ma J, Zheng J, Liu X, Liu Y, Ruan X et al (2020) Silencing SCAMP1-TV2 inhibited the malignant biological behaviors of breast cancer cells by interaction with PUM2 to facilitate INSM1 mRNA degradation. Front Oncol 10:613
Zou H, Luo J, Guo Y, Liu Y, Wang Y, Deng L et al (2022) RNA-binding protein complex LIN28/MSI2 enhances cancer stem cell-like properties by modulating Hippo-YAP1 signaling and independently of Let-7. Oncogene 41(11):1657–1672
Qin H, Li W, Sun Y, Bao Y, Sun L, Song Z et al (2018) 20(S)-25-methoxyl-dammarane-3β,12β,20-triol attenuates endoplasmic reticulum stress via ERK/MAPK signaling pathway. Eur J Pharmacol 836:75–82
Toyoda M, Suzuki D, Honma M, Uehara G, Sakai T, Umezono T et al (2004) High expression of PKC-MAPK pathway mRNAs correlates with glomerular lesions in human diabetic nephropathy. Kidney Int 66(3):1107–1114
Zhang J, Jing S, Zhang H, Zhang J, Xie H, Feng L (2022) Low-dose aspirin prevents LPS-induced preeclampsia-like phenotype via AQP-1 and the MAPK/ERK 1/2 pathway. Placenta 121:61–69
Salama AAA, Elgohary R, Fahmy MI (2023) Protocatechuic acid ameliorates lipopolysaccharide-induced kidney damage in mice via downregulation of TLR-4-mediated IKBKB/NF-kappaB and MAPK/Erk signaling pathways. J Appl Toxicol. https://doi.org/10.1002/jat.4447
Acknowledgements
We would like to give our sincere gratitude to the reviewers for their constructive comments.
Funding
This work was supported by Shandong Provincial Medical and Health Development Project (No. 202103060284) and the Research Fund for Academician Lin He New Medicine (No. JYHL2019FMS11).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors agree with the presented findings, have contributed to the work, and declare no conflict of interest.
Ethical approval
The animal studies were approved by the Animal Care and Use Committee of the Affiliated Hospital of Jining Medical University (JNMC-2020-DW-FY-041).
Informed consent disclosure
Not Applicable.
Additional information
This article belongs to the Topical Collection "Diabetic Nephropathy", managed by Giuseppe Pugliese.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
592_2023_2145_MOESM1_ESM.tif
Figure S1. HG induced HK2 cell apoptosis and ERS. HK2 cells were subjected to HG (15, 30 and 45 mM) for 24 h. HK-2 cell viability (A) and proliferation (B) were examined by MTT assay and EdU assay, respectively. C Flow cytometry was performed to examine HK2 cell apoptosis. D, E Bax, cleaved-caspase-3, Bcl-2, GRP78, CHOP and caspase12 protein levels were analyzed by western blot. The measurement data were presented as mean ± SD. All data was obtained from at least three replicate experiments. *p < 0.05, **p < 0.01, ***p < 0.001 (TIF 2815 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zheng, Y., Zhang, Z., Zheng, D. et al. METTL14 promotes the development of diabetic kidney disease by regulating m6A modification of TUG1. Acta Diabetol 60, 1567–1580 (2023). https://doi.org/10.1007/s00592-023-02145-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-023-02145-5