Abstract
Aim
To describe the development of the AWARE App, a novel web application for the rapid assessment of cardiovascular risk in Type 2 Diabetes Mellitus (T2DM) patients. We also tested the feasibility of using this App in clinical practice.
Methods
Based on 2019 European Society of Cardiology/European Association for the Study of Diabetes criteria for cardiovascular risk stratification in T2DM, the AWARE App classifies patients into very high (VHCVR), high (HCVR) and moderate (MCVR) cardiovascular risk categories. In this retrospective clinical study, we employed the App to assess the cardiovascular risk of T2DM patients, while also collecting data about current glycaemic control and pharmacological treatment.
Results
2243 T2DM consecutive patients were evaluated. 72.2% of the patients were VHCVR, 8.9% were HCVR, 0.8% were MCVR while 18.2% did not fit into any of the risk categories and were classified as “moderate-to-high” (MHCVR). Compared with the other groups, patients with VHCVD were more frequently ≥ 65 years old (68.9%), with a longer disease duration (≥ 10 years [56.8%]), a history of cardiovascular disease (41.4%), organ damage (35.5%) and a higher numbers of cardiovascular risk factors. Patients with MHCVD generally had disease duration < 10 years (96%), younger age (50–60 years [55%]), no history of cardiovascular disease, no organ damage, and 1–2 cardiovascular risk factors (89%). Novel drugs such as Glucagon Like Peptyde 1 Receptor Agonists or Sodium-Glucose Linked Transporter 2 inhibitors were prescribed only to 26.3% of the patients with VHCVR and to 24.7% of those with HCVR. Glycaemic control was unsatisfactory in this patients population (HbA1c 7.5 ± 3.4% [58.7 ± 13.4 mmol/mol]).
Conclusions
The AWARE App proved to be a practical tool for cardiovascular risk stratification of T2DM patients in real-world clinical practice.
Similar content being viewed by others
Introduction
Type 2 Diabetes Mellitus (T2DM) is a global health emergency. Its incidence and prevalence are exponentially increasing, particularly in developing countries [1].
T2DM micro- and macrovascular complications contribute substantially to the burden of the disease. They are major causes of increased morbidity and mortality, frequently resulting also in clinical and surgical emergencies [2, 3]. Clinical and epidemiological data have demonstrated that T2DM increases by 3–4 folds atherothrombotic risk, as well as chronic kidney disease risk [4, 5]. In T2DM, cardio-renal events are further boosted by the pathogenic link between ischemic heart disease and heart failure with renal disease [6].
Preventive and pharmacological interventions are essential to limit the growing burden of T2DM-related cardiovascular (CV) complications [7, 8]. In the last decade two new classes of anti-diabetic drugs proved to be particularly effective in attaining these goals: the Glucagon Like Peptide 1 Receptor Agonists (GLP-1 RA) and the Sodium-Glucose Co-Transporter-2 inhibitors (SGLT2i) [9,10,11,12,13,14,15,16,17,18,19,20,21]. The benefits of these two classes of drugs are not limited to glycaemic control since they also proved to be effective in reducing blood pressure [21, 22], body weight [23] and sub-inflammation [24, 25].
Clinical trials results with GLP-1 RA and SGLT2i have changed T2DM treatment paradigms, shifting the therapeutic target from glycaemic control to the possible prevention/slowing of organ damage and increased survival. This change of perspective has been implemented in recent guidelines developed jointly by diabetologists and cardiologists. In the European Society of Cardiology (ESC)/European Association for the Study of Diabetes (EASD) and in the ESC/European Association of Preventive Cardiology (EAPC) guidelines, GLP-1 RA and SGLT2i are included in pharmacological management algorithms as first-line treatment for patients with high or very high CV risk [26, 27].
This new approach requires a prompt and accurate assessment of each T2DM patient’s CV risk, which should then guide physicians in the choice of the best treatment options. Moreover, since CV risk can be regarded as a continuum, constantly changing over time, this risk should be assessed not only at the onset of the disease but also regularly during patients’ lifetime [28, 29]. This will allow taking into account the occurrence of intercurrent events (such as chronic diseases, cognitive decline, aging, coronary artery disease, stroke and loss of kidney function) which could change the CV risk level and thus prompt a treatment modification.
To guide physicians in assessing patients’ CV risk, the 2019 ESC-EASD guidelines introduced CV risk stratification criteria [26]. These guidelines proposed three risk categories (very high, high, and moderate) based on the presence of CV disease, other target organs damage (proteinuria, renal impairment defined as eGFR < 30 mL/min/1.73 m2, left ventricular hypertrophy, retinopathy), duration of disease, age, and/or presence of other known risk factors (arterial hypertension, dyslipidemia, smoking, obesity) [26].
Although ESC-EASD criteria are straightforward, their implementation may result unpractical in everyday clinical routine. This is particularly true in current diabetological practice landscape, where large numbers of patients and tight visit times may result in underutilization of this CV stratification tool and delay of organ-protective therapies initiation [30].
Technology may help overcome this obstacle. Today it is increasingly easy and unexpensive to build tailored small pieces of software which can simplify the execution of time-consuming tasks.
To this end, we developed the new AWARE App, a Web application that allows to assess CV risk according to the ESC/EASD criteria in about 20 s. The App was employed by a network of hospitals and outpatient clinics in Lombardy (Italy) to raise awareness about CV risk in T2DM and to simplify patient categorization.
This study aimed to test the feasibility of AWARE App use in routine clinical practice while also collecting real-world data about CV risk, glycaemic control and pharmacological treatment of patients with T2DM.
Methods
The AWARE app
AWARE is a Web App which runs on a Web server and can be loaded by any Internet browser, using both personal computers and mobile devices (smartphones, tablets). The AWARE App was developed by the Italian software house SoftwareVM on behalf of the Diabetes Centers involved in this study.
We named the App “AWARE” (raise AWAREness on the importance of CV risk assessment in T2DM patients) since we hypothesized that its use could help to choose the most appropriate antidiabetic medication according to each patient cardiovascular risk.
The main function of the AWARE App is the assessment of CV risk based on 2019 ESC/EASD criteria (Table 1). After loading, the App shows the main screen, which includes several options concerning the AWARE Project (Risk assessment, Patient report, Documents, About the project, and Working group). Currently, the only active option is “Risk assessment”: by clicking/touching it the CV risk assessment section is loaded. It consists in a short form which must be filled with some patient’s information such as age (< 50 or ≥ 50 years old), diabetes duration (< 10 or ≥ 10 years), presence of established CV disease (yes or no), organ damage (proteinuria, kidney disease, retinopathy, or LVH) and CV risk factors (smoke, dyslipidemia, hypertension, obesity, age; Supplementary Fig. 1). Once the form is filled, a button named “Assess risk” activates: by clicking/touching it, the App calculates and displays the patient’s level of CV risk. Only the age and the diabetes duration are mandatory informations required by the App; the other fields should be filled based on patient’s characteristics, but do not prevent the activation of the “Assess risk” button. The completion of the AWARE App Risk assessment form requires about 20 s, making the CV risk assessment fast and easy.
The AWARE App used in this study also recorded the patients’ level of glycated haemoglobin (HbA1c) and the prescribed class of medication, to provide further opportunities for data interpretation and future studies.
The AWARE App is free and available online in English language at the following URL: https://aware.softwarevm.online/ (user ID: Aware; password: Aware).
Study design and participants
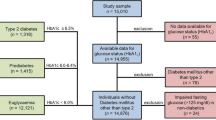
This was a retrospective, observational, multi-center study, conducted by a network of Diabetes Centers in Lombardy (Italy). 2243 consecutive T2DM patients attending the Centers from November 2020 to April 2021 were enrolled. The AWARE App was used to calculate each patient’s CV risk and to record his/her HbA1c level and current pharmacological treatment. Anonymized data were stored on the App Web server and retrospectively analysed. The study protocol was approved by the IRCSS MultiMedica, Sesto San Giovanni (MI), Italy, Ethics Committee (Protocol n. 498.2021 approved on 10/03/2022).
Statistical analysis
Continuous variables are reported as mean and standard deviation (SD), while categorical variables are reported both as absolute numbers and percentages representing relative prevalence. Differences between groups were analysed with the Chi-squared test or Fischer's exact test for categorical variables and with Student t-test or Mann–Whitney U-test for continuous variables, as appropriate. A one-way analysis of variance (ANOVA) was performed to compare differences among groups in continuous variables. A P value < 0.05 was considered statistically significant. All the analyses were performed with STATA 12.1 (Statistics/Data Analysis, Stata Corp, College Station, Texas).
Results
Overall, 2243 T2DM patients underwent CV risk assessment with the AWARE App and were included in this analysis. The majority of these subjects (n = 1619 [72.2%]) had a very high CV risk (VHCVR), 199 (8.9%) a high CV risk (HCVR), and only 17 (0.8%) a moderate CV risk (MCVR) (Table 2).
Interestingly, 408 of the patients (18.2%) did not fit into any of the 3 ESC/EASD risk categories. Most of the patients in this subgroup (n = 284 [69.6%]) were > 50 years old and with T2DM duration < 10 years, while only 8.8% of them did not present any CV risk factor. Since their characteristics were intermediate between MCVR and HCVR categories, we classified these patients into a newly coined additional CV risk category which we called “moderate-to-high” (MHCVR).
CV risk factors
41.4% (n = 671) of the patients with VHCVR had a history of established CV disease, 35.9% (n = 582) other target organ damage, 16.9% (n = 273) proteinuria, 16.2% (n = 263) retinopathy, and 11.4% (n = 185) reduced eGFR (eGFR ≤ 30 ml/min/1.73 m2). Compared with other risk groups, patients with VHCVR were more frequently smokers (15.9% vs. 3.0%, 0% and 9.6% in patients with HCVR, MCVR, and MHCVR, respectively; p < 0.001), dyslipidemic (76.3% vs. 38.2%, 0%, and 40.4%; p < 0.001), hypertensive (87.3% vs. 50.3, 0%, and 45.6%; p < 0.001), and obese (43.3% vs. 15.6%, 0%, and 27.2%; p < 0.001).
Younger patients (< 50 years old) were almost evenly distributed between the MHCVR and VHCVR categories (45.3% [n = 112] and 43.7% [n = 108] respectively), while the vast majority of those aged ≥ 65 had VHCVR (85.4% [n = 1115]).
Compared with other risk groups, patients with VHCVR were older, with the highest rate of age ≥ 65 (68.9% vs. 59.3%, 0%, and 17.9% in patients with HCVR, MCVR and MHCVR, respectively; p < 0.001), and duration of T2DM ≥ 10 years (56.8% vs. 0%, 0%, and 4.4%; p < 0.001).
T2DM pharmacological treatments
This analysis showed significant differences in pharmacological treatment between different CV risk groups (Table 2).
Compared with other risk groups, patients with VHCVR showed the highest rate of treatment with basal insulin (32.4% vs. 26.6%, 11.8%, and 12% in patients with HCVR, MCVR, and MHCVR, respectively; p < 0.001), rapid insulin analogues (13.6% vs. 12.6%, 0%, and 5.6%; p = 0.019) and GLP-1 RA (13.8% vs. 11.6% vs. 0%, and 10%; p = 0.007). Notably, GLP-1 RA or SGLT-2i were prescribed only to 26.3% of the patients with VHCVR and to 24.7% of those with HCVR.
Patients with HCVR had the highest rate of treatment with DPP4i (23.6% vs. 15.5%, 17.6%, and 12.5% in patients with VHCVR, MCVR, and MHCVR, respectively; p = 0.005) and sulfonylureas (14.6% vs. 9.9% vs. 11.8% vs. 6.6%; p = 0.002), while patients with MCVR showed the highest rate of pioglitazone utilization (11.8% vs. 5.5%, 9,0, and 3.2% in patients with VHCVR, HCVR, and MHCVR, respectively; p = 0.016).
Glycaemic control
The mean HbA1c level of the study population was 7.5 ± 3.4% (58.7 ± 13.4 mmol/mol) and this analysis showed significant differences in glycaemic control in the 4 CV risk groups (p = 0.007). HbA1c level was 8.1 ± 4.1% (65.3 ± 21.6 mmol/mol) in patients with MCVR, 7.4 ± 3.5% (57.0 ± 14.3 mmol/mol) in patients with MHCVR, 7.5 ± 3.4% (58.2 ± 13.5 mmol/mol) in patients with HCVR, and 7.6 ± 3.3% (59.1 ± 13.1 mmol/mol) in patients with VHCVR (p = 0.007).
Figure 1a shows the distribution of HbA1c measurements in each CV risk group. According to the 2022 American Diabetes Association (ADA) Standards of Medical Care in Diabetes, the appropriate HbA1c target for T2DM treatment in non-pregnant adults without significant hypoglycaemia is < 7% (< 53 mmol/mol) [31]. Therefore, only 26.7% (n = 599) of the all population achieved ADA glycaemic target. In particular, the percentage of patients at target was higher (p < 0.01) in patients with MCVD (35.3%) and MHCVD (34.8%) as compared with HCVD (26.1%) and VHCVD (24.6%) (Fig. 1b).
HbA1c levels distribution in the study population. a HbA1c values plotted as individual dots, and mean values ± 95% CI are shown for each CV risk group (moderate-to-high [light blue], moderate [blue], high [orange], very high [red]) and for the total populations (gray). The dashed line displays the 2022 ADA guidelines HbA1c target (53 mmol/mol [7%]), which was reached only by the 26.7% of the tested patients. b Bars represent the number (%) ± SD of patients with HbA1c levels lower (left panel) or higher (right panel) than 53 mmol/mol (7%) in moderate-to-high (light blue), moderate (blue), high (orange), and very high (red) groups, showing that patients classified in the moderate-to-high and moderate groups have statistically significant lower HbA1c levels (** = p < 0.01; *** = p < 0.001;**** = p < 0.0001. HbA1c: glycated hemoglobin; CI: confidence interval; CV: cardiovascular; ADA: American Diabetes Association; SD: standard deviation)
We also evaluated whether the presence or absence of all the recorded patients’ characteristics was associated with higher or lower levels of HbA1c (Table 3). HbA1c levels were significantly higher in patients with established CVD (p = 0.041), longer diabetes duration (≥ 10 vs < 10 years; p < 0.001), younger age (< 65 vs. ≥ 65 years; p = 0.013), retinopathy (p < 0.001), dyslipidemia (p < 0.001), arterial hypertension (p = 0.007), and obesity (BMI ≥ 30 kg/m2; p < 0.001).
HbA1c levels were significantly lower in patients without CV risk factors compared with subjects with at least one risk factor (p = 0.001), with glycaemic control progressively worsening with the increase of risk factors number.
Similarly, the presence of target organ damage was associated with higher HbA1c levels (p = 0.003), with worsening of glycaemic control being associated with the increased number of affected organs.
Finally, the type of pharmacological treatment seemed to be associated with different glycaemic control. Patients treated with insulin or sulfonylureas showed higher HbA1c values (p < 0.001) as compared with patients treated with the newer antidiabetic medications (DPP4i, GLP-1 RA, and SGLT2i) which had better glycaemic control (p = 0.002, p = 0.010, and p = 0.008 respectively).
Discussion
To the best of our knowledge, this is the first study to test a Web App for T2DM patients’ CV risk stratification. In our experience, the AWARE App proved to be a suitable tool for real-world clinical practice and it allowed us to assess rapidly and efficiently in more than 2,000 consecutive subjects.
The T2DM CV risk redefinition introduced in 2019 ESC/EASD guidelines, based on risk factors, organ damage, and duration of disease, places a large proportion of patients in the high and very high risk categories. This was confirmed by our study since the majority of the enrolled patients (72.2%) belonged to the VHCVR group. These patients were generally ≥ 65 years old (68.9%), with a long disease duration (≥ 10 years [56.8%]), a history of established CV disease (41.4%) and organ damage (35.5%). As expected, patients with VHCVR also showed higher rates of hypertension, dyslipidaemia and obesity as compared with other risk groups.
Interestingly, about 18% of the patients in this study did not fit in any of the three 2019 ESC/EASD CV risk categories and we included them in the newly coined MHCVR group. The majority of the subjects in this group had shorter disease duration (< 10 years) and younger age (50–65 years). None of them had a history of CV disease or was affected by target organ damage such as retinopathy, proteinuria and advanced kidney disease. However, almost 90% of the MHCVR patients had one or more CV risk factors, and their rates of hypertension, dyslipidaemia, and obesity were higher compared with those of patients with HCVR. In our opinion, this relatively young population, with short disease duration, less than 3 CV risk factors, that has not already developed any retinal or cardio-renal complication, may also greatly benefit from newer therapies such as GLP-1 RA and SGLT2i, which have demonstrated to reduce CV diseases risk and mortality [32, 33].
This study results are consistent with published data. A recent prospective study conducted on 1,690 T2DM patients compared the prognostic performance of the 2019 ESC/EASD CV risk model with the Systematic COronary Risk Evaluation (SCORE) risk model and N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels measurement [34]. The high rate of patients belonging to the 2019 ESC/EASD very high risk category and the rate of subjects who could not be categorized according to these criteria were similar to our findings (66% and 17%, respectively). Interestingly, the uncategorized patients’ characteristics were similar to those of our MHCVR subjects in terms of clinical features, such as disease duration (< 10 years) and number of CV risk factors (< 3).
In an Italian, retrospective study, which evaluated 473,740 T2DM patients, the rate of very high CV risk subjects based on 2019 ESC/EASD criteria resulted similar to our findings (78.5%) [35]. The characteristics of this subgroup of patients were consistent with our data (long disease duration, history of CV events, high rates of end-organ damage and ≥ 3 CV risk factors). According to the authors of this study, T2DM is not always an equivalent of CV risk (i.e. T2DM patients without coronary artery disease [CHD] and patients with only CHD have similar mortality rates); in fact, it could exist a subgroup of younger patients with shorter disease duration and with low risk of CV events [36]. This subgroup of T2DM patients, with features similar to those of our MHCVR subjects, could not be categorized employing the 2019 ESC/EASD criteria, and may therefore not receive the most appropriate treatment.
Even though knowing the CV risk level is essential for choosing the most appropriate treatment in T2DM patients, the degree of glycometabolic control is critically important. This is the reason why we decided to include in the AWARE App form also the HbA1c level (which is not included in the 2019 ESC/EASD criteria). In our study population, glycaemic control was unsatisfactory, with HbA1c level of 7.5 ± 3.4% (58.7 ± 13.4 mmol/mol) and a high proportion (almost 3/4) of patients with HbA1c values above the 2022 ADA Standard of medical Care in Diabetes and 2019 ESC/EASD guidelines recommended target (< 53 mmol/mol, < 7%) [26, 31]. This result may be in part due to delayed choice of treatment (as shown by the low rate of patients with VHCVR treated with GLP-1 RA or SGLT2i), and it is consistent with the findings of the CAPTURE study. In this multinational, cross-sectional trial, conducted in 9,823 T2DM patients and 13 different countries, the median HbA1c level was 7.3% (6.6–8.4%) (56 mmol/mol [49–68 mmol/mol]) and only 21.5% of the patients with established CV disease were treated with GLP-1 RA of SGLT2i [37].
The unsatisfactory glycaemic control reported in our study may also reflect a cultural legacy stemming from trials such as ACCORD and VADT, which demonstrated that intensive glycaemic control in T2DM patients does not provide any significant benefit and can even increase mortality [38]. It should be highlighted, though, that ACCORD and VADT patients were older, with longstanding diabetes, and a great prevalence of macrovascular disease; in subjects with these characteristics (which are similar to those of the VHCVR group in our study), strictly pursuing near normal glucose levels with insulin could increase the frequency of hypoglycaemic events, a strong risk factor for CV acute complications and sudden death. On the contrary, UKPDS clearly showed that younger patients, with early diabetes and no overt CD disease (similar to those of our MHCVR group), can greatly benefit from more aggressive glucose management in terms of long-term reduction of myocardial infarction, death from any cause, and microvascular disease[38]. Moreover, ACCORD, VADT and UKPDS results were obtained with conventional antidiabetic drugs and the discovery of newer treatments requires a re-appraisal of those findings. Thanks to the intrinsic low risk of hypoglycaemia associated with GLP-1 RA of SGLT2i, these drugs allow to safely achieve the recommended HbA1c levels, even in patients with advanced diabetes, thus allowing more aggressive treatment of T2DM. Recently, several small studies in newly diagnosed T2DM patients showed that the use of a combination of multiple drugs with complementary mechanisms of action (metformin, pioglitazone, and exenatide) provides better outcomes compared with the sequential treatment with conventional medications [39,40,41].
In conclusion, we believe that the use of the web app AWARE to evaluate CV risk and implement more aggressive earlier treatment with newer medications could represent a step forward to help preventing chronic severe and invalidating complications and premature death in T2DM. These hypothesis should be verified by larger, prospective trials, in order to possibly overcome two limitations of our study, i.e. 1) its retrospective design and 2) a sample of patients belonging to a restricted geographical region.
Conclusion
The AWARE App represents a practical tool for a very rapid CV risk stratification of T2DM patients, with the potential to increase physicians’ awareness of this important patient feature, to guide them in the choice of the best therapeutic option, and improve their adherence to current treatment guidelines. In our population, the stratification with the AWARE App showed a vast majority of T2DM patients with very high CV risk and a relevant subgroup (about 20%) who did not fit in any 2019 ESC/EASD category. Moreover, it showed low treatment’s rates with newer T2DM drugs (GLP-1 RA and SGLT2i) in patients with high or very high CV risk which might benefit from these treatments.
Data availability
The dataset analysed in the current study will be made available by the corresponding authors upon reasonable request.
References
Liu J, Ren ZH, Qiang H, Wu J, Shen M, Zhang L et al (2020) Trends in the incidence of diabetes mellitus: results from the global burden of disease study 2017 and implications for diabetes mellitus prevention. BMC Public Health 20(1):1415. https://doi.org/10.1186/s12889-020-09502-x
Deshpande AD, Harris-Hayes M, Schootman M (2008) Epidemiology of diabetes and diabetes-related complications. Phys Ther 88(11):1254–1264. https://doi.org/10.2522/ptj.20080020
Harding JL, Pavkov ME, Magliano DJ, Shaw JE, Gregg EW (2019) Global trends in diabetes complications: a review of current evidence. Diabetologia 62(1):3–16. https://doi.org/10.1007/s00125-018-4711-2
Emerging Risk Factors Collaboration, Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E et al (2010) Diabetes mellitus fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 375(9733):2215–2222. https://doi.org/10.1016/S0140-6736(10)60484-9
Vesa CM, Popa L, Popa AR, Rus M, Zaha AA, Bungau S et al (2020) Current data regarding the relationship between type 2 diabetes mellitus and cardiovascular risk factors. Diagnostics (Basel) 10(5):314. https://doi.org/10.3390/diagnostics10050314
Banerjee S, Panas R (2017) Diabetes and cardiorenal syndrome: understanding the Triple Threat. Hellenic J Cardiol 58(5):342–347. https://doi.org/10.1016/j.hjc.2017.01.003
Gruss SM, Nhim K, Gregg E, Bell M, Luman E, Albright A (2019) Public health approaches to type 2 diabetes prevention: the US national diabetes prevention program and beyond. Curr Diab Rep 19(9):78. https://doi.org/10.1007/s11892-019-1200-z
Coccheri S (2007) Approaches to prevention of cardiovascular complications and events in diabetes mellitus. Drugs 67(7):997–1026. https://doi.org/10.2165/00003495-200767070-00005
Brown E, Heerspink HJ, Cuthbertson DJ, Wilding JP (2021) SGLT2 Inhibitors and GLP-1 receptor agonists: established and emerging indications. Lancet 398(10296):262–276. https://doi.org/10.1016/S0140-6736(21)00536-5
Zinman B, Wanner C, Lachin JM, Fitchett D, BluMHki E, Hantel S et al (2015) EMPA-REG OUTCOME Investigators empagliflozin cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 373(22):2117–2128. https://doi.org/10.1056/NEJMoa1504720
Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJ, Charytan DM et al (2019) CREDENCE Trial investigators Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 380(24):2295–2306. https://doi.org/10.1056/NEJMoa1811744
Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M et al (2016) EMPA-REG OUTCOME Investigators empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 375(4):323–334. https://doi.org/10.1056/NEJMoa1515920
Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A et al (2019) DECLARE–TIMI 58 Investigators Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 380(4):347–357. https://doi.org/10.1056/NEJMoa1812389
McMurray JJ, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA et al (2019) DAPA-HF Trial committees and Investigators Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 381(21):1995–2008. https://doi.org/10.1056/NEJMoa1911303
Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA et al (2016) LEADER Trial investigators Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 375(4):311–322. https://doi.org/10.1056/NEJMoa1603827
Lapolla A, Berra C, Boemi M, Bossi AC, Candido R, Di Cianni G et al (2018) NN2211–4118 Study group Long-term effectiveness of liraglutide for treatment of type 2 diabetes in a real-Life setting: a 24-month multicenter non-interventional retrospective study. Adv Ther 35(2):243–253. https://doi.org/10.1007/s12325-017-0652-2
Mirani M, Favacchio G, Serone E, Lucisano G, Rossi MC, Berra CC (2018) Liraglutide and cardiovascular outcomes in a real world type 2 diabetes cohort. Pharmacol Res 137:270–279. https://doi.org/10.1016/j.phrs.2018.09.003
Jabbour SA, Frías JP, Hardy E, AMHed A, Wang H, ÖMHan P et al (2018) Safety and efficacy of exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy: 52-week results of the DURATION-8 randomized controlled trial. Diabetes Care 41(10):2136–2146. https://doi.org/10.2337/dc18-0680
Gerstein HC, Colhoun MH, Dagenais GR, Diaz R, LaksMHanan M, Pais P et al (2019) REWIND Investigators Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind randomised placebo-controlled trial. Lancet 394(10193):121–130. https://doi.org/10.1016/S0140-6736(19)31149-3
Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA et al (2016) SUSTAIN-6 Investigators semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 375(19):1834–1844. https://doi.org/10.1056/NEJMoa1607141
Sattar N, Lee MM, Kristensen SL, Branch KR, Del Prato S, Khurmi NS et al (2021) Cardiovascular mortality and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol 9(10):653–662. https://doi.org/10.1016/S2213-8587(21)00203-5
Berra C, Manfrini R, Regazzoli D, Radaelli MG, Disoteo O, Sommese C et al (2020) Blood pressure control in type 2 diabetes mellitus with arterial hypertension. The important ancillary role of SGLT2-inhibitors and GLP1-receptor agonists. Pharmacol Res 160:105052. https://doi.org/10.1016/j.phrs.2020.105052
Lazzaroni E, Ben Nasr M, Loretelli C, Pastore I, Plebani L, Lunati ME et al (2021) Anti-diabetic drugs and weight loss in patients with type 2 diabetes. Pharmacol Res 171:105782. https://doi.org/10.1016/j.phrs.2021.105782
Bendotti G, Montefusco L, Lunati ME, Usuelli V, Pastore I, Lazzaroni E et al (2022) The anti-inflammatory and immunological properties of GLP-1 receptor agonists. Pharmacol Res 182:106320. https://doi.org/10.1016/j.phrs.2022.106320
La Grotta R, de Candia P, Olivieri F, Matacchione G, Giuliani A, Rippo MR et al (2022) Anti-inflammatory effect of SGLT-2 inhibitors via uric acid and insulin. Cell Mol Life Sci 79(5):273. https://doi.org/10.1007/s00018-022-04289-z
Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V et al (2019) ESC Scientific Document Group 2019 ESC guidelines on diabetes pre-diabetes and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 41(2):255–323. https://doi.org/10.1093/eurheartj/ehz486
Visseren FL, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M et al (2021) ESC National cardiac societies ESC scientific document group ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 42(34):3227–3337. https://doi.org/10.1093/eurheartj/ehab484
Hsueh WA, Law RE (1998) Cardiovascular risk continuum: implications of insulin resistance and diabetes. Am J Med 105(1A):4S-14S. https://doi.org/10.1016/s0002-9343(98)00205-8
Williams BA, Blankenship JC, Voyce S, Cordova JM, Gandhi P, Shetty SS (2021) Quantifying the risk continuum for cardiovascular death in adults with type 2 diabetes. Can J Diabetes 45(7):650-658.e2. https://doi.org/10.1016/j.jcjd.2021.01.009
Dhippayom T, Chaiyakunapruk N, Krass I (2014) How diabetes risk assessment tools are implemented in practice: a systematic review. Diabetes Res Clin Pract 104(3):329–342. https://doi.org/10.1016/j.diabres.2014.01.008
American Diabetes Association Professional Practice Committee, Draznin B, Aroda VR, Bakris G, Benson G, Brown FM, Freeman R, et al. (2022) 6. Glycemic Targets: Standards of Medical Care in Diabetes-2022. Diabetes Care. 45(Suppl 1):S83-S96. https://doi.org/10.2337/dc22-S006.
McGuire DK, Shih WJ, Cosentino F, Charbonnel B, Cherney DZ, Dagogo-Jack S et al (2021) Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol 6(2):148–158. https://doi.org/10.1001/jamacardio.2020.4511
De Block C (2018) SGLT2 inhibitors and GLP-1 receptor agonists: a sound combination. Lancet Diabetes Endocrinol 6(5):349–352. https://doi.org/10.1016/S2213-8587(18)30031-7
Prausmüller S, Resl M, Arfsten H, Spinka G, Wurm R, Neuhold S et al (2021) Performance of the recommended ESC/EASD cardiovascular risk stratification model in comparison to SCORE and NT-proBNP as a single biomarker for risk prediction in type 2 diabetes mellitus. Cardiovasc Diabetol 20(1):34. https://doi.org/10.1186/s12933-021-01221-w
Pintaudi B, Scatena A, Piscitelli G, Frison V, Corrao S, Manicardi V et al (2021) Clinical profiles and quality of care of subjects with type 2 diabetes according to their cardiovascular risk: an observational, retrospective study. Cardiovasc Diabetol 20:59. https://doi.org/10.1186/s12933-021-01251-4
Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M (1998) Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 339(4):229–234. https://doi.org/10.1056/NEJM199807233390404
Mosenzon O, Alguwaihes A, Leon JL, Bayram F, Darmon P, Davis TM et al (2021) CAPTURE: a multinational cross-sectional study of cardiovascular disease prevalence in adults with type 2 diabetes across 13 countries. Cardiovasc Diabetol 20(1):154. https://doi.org/10.1186/s12933-021-01344-0
Terry T, Raravikar K, Chokrungvaranon N, Reaven PD (2012) Does aggressive glycemic control benefit macrovascular and microvascular disease in type 2 diabetes? Insights from ACCORD, ADVANCE, and VADT. Curr Cardiol Rep 14(1):79–88. https://doi.org/10.1007/s11886-011-0238-6
Lavynenko O, Abdul-Ghani M, Alatrach M, Puckett C, Adams J, Abdelgani S et al (2022) Combination therapy with pioglitazone/exenatide/metformin reduces the prevalence of hepatic fibrosis and steatosis: the efficacy and durability of initial combination therapy for type 2 diabetes (EDICT). Diabetes Obes Metab 24(5):899–907. https://doi.org/10.1111/dom.14650
Abdul-Ghani MA, Puckett C, Triplitt C, Maggs D, Adams J, Cersosimo E et al (2015) Initial combination therapy with metformin pioglitazone and exenatide is more effective than sequential add-on therapy in subjects with new-onset diabetes. results from the efficacy and durability of initial combination therapy for type 2 Diabetes (EDICT): a randomized trial. Diabetes Obes Metab 17(3):268–275. https://doi.org/10.1111/dom.12417
Abdul-Ghani M, Puckett C, Adams J, Khattab A, Baskoy G, Cersosimo E et al (2021) Durability of triple combination therapy versus stepwise addition therapy in patients with new-onset T2DM: 3-year follow-up of EDICT. Diabetes Care 44(2):433–439. https://doi.org/10.2337/dc20-097
Acknowledgements
The authors thank Drs. Andrea Bolla, Barbara Guazzini, Elena Cimino, Elena Passeri, Fabrizia Ida Pastore, Giuseppina De Felice, Laura Molteni, Milena Muratori, Paola Bollati, and Rosa Terranova for their support in the conduct of the study.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
CB designed the study and wrote the manuscript. MM and FF contributed to the results interpretation and wrote the manuscript. FF and PF critically reviewed the manuscript. LB, MEL, RG, and RM conducted the investigation. ASZ and FB performed statistical analyses. SP, SR, and FB contributed to the results interpretation.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Informed consent
Written informed consent was obtained from all study participants in accordance with the guidelines of the Declaration of Helsinki.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study protocol was approved by the IRCSS MultiMedica Ethical Committee.
Additional information
Managed by Massimo Federici.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Berra, C., Manfrini, R., Mirani, M. et al. AWARE A novel web application to rapidly assess cardiovascular risk in type 2 diabetes mellitus. Acta Diabetol 60, 1257–1266 (2023). https://doi.org/10.1007/s00592-023-02115-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-023-02115-x