Abstract
Aim
To compare HbA1c and clinical outcomes in adolescents and young adults with type 1 diabetes (T1D), with or without celiac disease (CD).
Methods
Longitudinal data were extracted from ADDN, a prospective clinical diabetes registry. Inclusion criteria were T1D (with or without CD), ≥ 1 HbA1c measurement, age 16–25 years and diabetes duration ≥ 1 year at last measurement. Multivariable Generalised Estimated Equation models were used for longitudinal analysis of variables associated with HbA1c.
Results
Across all measurements, those with coexisting T1D and CD had lower HbA1c when compared to those with T1D alone (8.5 ± 1.5% (69.4 ± 16.8 mmol/mol) vs. 8.7 ± 1.8% (71.4 ± 19.8 mmol/mol); p < 0.001); lower HbA1c was associated with shorter diabetes duration (B = − 0.06; 95% CI − 0.07 to − 0.05; p < 0.001), male sex (B = − 0.24; − 0.36 to − 0.11; p < 0.001), insulin pump therapy use (B = − 0.46; − 0.58 to − 0.34; p < 0.001), coexistence of T1D and CD (B = − 0.28; − 0.48 to − 0.07; p = 0.01), blood pressure (B = − 0.16; − 0.23 to − 0.09; p < 0.001) and body mass index (B = -− 0.03; − 0.02 to − 0.04; p = 0.01) in the normal range. At last measurement, 11.7% of the total population had a HbA1c < 7.0% (53.0 mmol/mol).
Conclusions
Across all measurements, coexisting T1D and CD is associated with lower HbA1c when compared to T1D alone. However, HbA1c is above target in both groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The association between type 1 diabetes and celiac disease (CD) is well documented, with the estimated prevalence of CD in people with type 1 diabetes varying worldwide. For example, in 57,375 people with type 1 diabetes from the Better Control in Pediatric and Adolescent Diabetes: Working to Create Centers of Reference (SWEET) registry, CD prevalence was 4.5%, with different prevalence among regions: from 1.9% in Asia/Middle East to 6.9% in Australia/New Zealand [1]. Elsewhere, recent data demonstrate a CD prevalence of 5.6% in children and adolescents with type 1 diabetes from Sweden [2], and in 52,721 people from the Prospective Diabetes Follow-up Registry (DPV) (Germany/Austria), the T1D Exchange Clinic Network (T1DX) (United States of America), the National Paediatric Diabetes Audit (NPDA) (United Kingdom [England/Wales]) and the Australasian Diabetes Data Network (ADDN) (Australia), the prevalence of CD in children and adolescents with type 1 diabetes was 3.2%, 1.9%, 3.8% and 7.7%, respectively [3]. Although most prevalence studies only include children and adolescents, figures are similar when extended to young adults (5.0%) [4], much higher than the pooled prevalence of 1.4% in the general population [5]; case detection being likely to reflect screening practices within the type 1 diabetes population and in at-risk cohorts.
Adverse effects of untreated CD include iron deficiency, anemia, abnormal cortical and trabecular bone, osteoporosis and growth retardation [6]. Some studies have demonstrated that the coexistence of type 1 diabetes and CD is associated with higher HbA1c, as well as an increased risk of hypoglycemia and microvascular complications [7,8,9]. Conversely, other studies have shown no difference or lower HbA1c and lower incidence of complications [3, 10,11,12,13,14]; a variation possibly occurring, in part, due to consideration around adherence to a gluten free diet in sample populations [14, 15].
There are limited data on HbA1c or therapy among adolescents and young adults with coexisting type 1 diabetes and CD [11, 12, 14]; this age range is important as it represents a distinct phase of maturation, and it is widely documented that in type 1 diabetes, HbA1c is typically higher in this population [16,17,18]. We hypothesized that HbA1c would be comparable among adolescents and young adults with type 1 diabetes, with or without CD.
Methods
Population
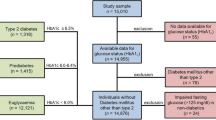
We used prospectively collected longitudinal data from the Australasian Diabetes Data Network (ADDN), a research collaboration among Australasian diabetes centers [19]. Inclusion criteria were type 1 diabetes (with or without CD), ≥ 1 HbA1c measurement, and at the last HbA1c measurement of age 16–25 years with diabetes duration ≥ 1 year; an age range chosen as it covers transition from paediatric to adult services, and because maturation is variably but generally completed by age 25 years [16]. Data extracted (June 1997 to March 2021) included socio-demographic variables such as sex, country of birth, self-identified Indigenous status and center, and clinical variables such as age at visit, CD diagnosis, date of visit, HbA1c, insulin regimen (twice daily injection (BD), multiple daily injection (MDI) or continuous subcutaneous insulin infusion (CSII)), total daily dosage (TDD) of insulin, blood pressure (BP) and body mass index (BMI).
Definitions and approvals
BP was defined as being in hypertensive ranges when participants aged < 18 years had a systolic and/or diastolic BP at ≥ 95th percentile and, for those aged ≥ 18 years, systolic BP ≥ 130 and/or diastolic BP ≥ 80 mmHg [20]. For those aged < 18 years, standardized BMI scores were based on Centers for Disease Control and Prevention reference data [21]. Overweight/obesity was defined as BMI standard deviation score (SDS) ≥ 85th percentile for those aged < 18 years or BMI > 25 kg/m2 for those ≥ 18 years. All centers had Human Research or Health and Disability Ethics Committee approval for participation in ADDN, and the current analysis was approved by the University of the Sunshine Coast Human Research Ethics Committee, Australia (reference: E19116).
Statistical methods
Descriptive statistics are reported as mean ± SD or number (%). HbA1c values were categorized into < or ≥ 7.0% (53.0 mmol/mol) based upon the International Society for Pediatric and Adolescent Diabetes (ISPAD) target of < 7.0% (< 53.0 mmol/mol) [22] and < or ≥ 9.0% (75.0 mmol/mol), a value widely considered to be a marker of very suboptimal glycemia. Results are stratified by presence or absence of CD, with differences between groups examined using Chi-square and t-tests. Generalized Estimated Equation (GEE) modelling was used for multivariable longitudinal analyses of HbA1c measurements across all visits. Explanatory variables included in the models were: type 1 diabetes duration, sex (male vs. female), presence or absence of CD, CSII (vs. BD/MDI) therapy, BP measurement in the hypertensive range (no/yes), and elevated BMI (no/yes); variables chosen based on clinical knowledge and previous literature. Goodness of fit was assessed using the two extensions of Akaike’s information criterion for model selection: quasi-likelihood under the independence model criterion (QIC) for choosing the best correlation structure and another QIC measure for choosing the best subset of predictors.
Results are reported as beta and 95% confidence intervals; p < 0.05 was considered significant and all assumptions were tested and met. Age at visit was not used in GEE models due to collinearity with diabetes duration. Analyses were performed using SPSS version 29™ software (IBM, New York).
Results
Last HbA1c measurement
A cohort of 6,480 adolescents and young adults with type 1 diabetes (52.6% male) met the inclusion criteria; data heralded from 24 centers across Australia and New Zealand (13 pediatric and 11 adult). Mean age was 18.3 ± 2.2 years, age at type 1 diabetes diagnosis was 9.1 ± 4.4 years, and diabetes duration was 8.7 ± 4.6 years. Place of birth was Australia or its territories in 85.1%, and New Zealand in 5.5%; 51 (1.2%) identified as Aboriginal and 66 (1.6%) as Māori. Most (52.2%) utilized MDI therapy, with 38.7% using CSII and 9.1% using BD injections; mean HbA1c was 8.8% ± 1.4 (72.9 ± 20.5 mmol/mol). In total, 362 (5.6%) adolescents and young adults had co-existing CD. Mean age at CD diagnosis was 10.2 ± 3.6 years, with CD diagnosed 2.0 ± 3.8 years after a type 1 diabetes diagnosis.
Characteristics of those with coexisting type 1 diabetes and CD vs. type 1 diabetes alone at last HbA1c measurement are shown in Table 1. Those with coexisting type 1 diabetes and CD were more likely to be female, younger, diagnosed with type 1 diabetes at a younger age and attend a pediatric center. Mean HbA1c did not significantly differ between those with coexisting type 1 diabetes and CD vs. type 1 diabetes alone; only 11.7% of the total population had a HbA1c < 7.0% (53.0 mmol/mol); 38.5% had HbA1c ≥ 9.0% (75.0 mmol/mol). The proportions that had a HbA1c measurement categorized as either < 7.0% (53.0 mmol/mol), 7.0–8.9% (53.0–75.0 mmol/mol) or ≥ 9.0% (75.0 mmol/mol) differed (p = 0.01), as did the proportions using BD, MDI or CSII therapy (p < 0.001). Adolescents and young adults with type 1 diabetes and coexisting CD had a trend towards higher TDD of insulin, less BP measurements in the hypertensive range and elevated BMI.
Values of HbA1c at last measurement stratified by type 1 diabetes duration and presence or absence of CD are shown in Fig. 1; generally, as type 1 diabetes duration increased, the proportion of adolescents and young adults achieving an HbA1c < 7.0% (53.0 mmol/mol) decreased.
All HbA1c measurements
Across all HbA1c measurements (n = 32,025) across all visits, there was a small but significant difference in the number of measurements in those with co-existing type 1 diabetes and CD vs. type 1 diabetes alone (5.5 ± 4.1 vs. 4.7 ± 3.8; p < 0.001). Mean HbA1c across all visits was 8.7 ± 1.8% (71.2 ± 20.0 mmol/mol) and was slightly lower in those with coexisting type 1 diabetes and CD (8.5 ± 1.5% (69.4 ± 16.8 mmol/mol) vs. 8.7 ± 1.8% (71.4 ± 19.8 mmol/mol); p < 0.001).
Using multivariable GEE modelling, lower HbA1c was associated with shorter type 1 diabetes duration, male sex, use of CSII therapy, coexistence of type 1 diabetes and CD, and BP and BMI in the normal range (Table 2). In subgroup analysis of those with coexisting diabetes and CD, lower HbA1c was associated with shorter type 1 diabetes duration (B = − 0.09, 95% CI = − 0.13 to − 0.04; p < 0.001), and use of CSII therapy (B = − 0.46, − 0.81 to − 0.12; p < 0.001).
Discussion
Our findings indicate that coexisting type 1 diabetes and CD is associated with lower HbA1c when compared to those with type 1 diabetes alone. Across all HbA1c measurements, those with coexisting type 1 diabetes and CD had a small but statistically significantly lower HbA1c; lower HbA1c was associated with shorter type 1 diabetes duration, male sex, use of CSII therapy, coexistence of type 1 diabetes and CD, and BP and BMI in the normal range. However, HbA1c remains above target in both groups. It is disappointing that, at last HbA1c measurement, only 11.7% of the total population had a HbA1c < 7.0% (53.0 mmol/mol), with 38.5% having a HbA1c ≥ 9.0% (75.0 mmol/mol).
Findings relating to glycemia in those with coexisting type 1 diabetes and CD are consistent with data in youth in the ADDN registry [17]. In our previous report, mean HbA1c was 8.8 ± 1.8% (72.2 ± 19.9 mmol/mol) and only 12.3% had an HbA1c < 7.0% (53 mmol/mol); lower HbA1c was associated with male sex and use of CSII therapy, with higher HbA1c associated with longer type 1 diabetes duration. However, when considering the impact of coexisting CD, findings differ to elsewhere in the literature. In youth with type 1 diabetes across three international registries, we have demonstrated that HbA1c was comparable [3].
ISPAD recommend screening for CD in young people at type 1 diabetes diagnosis and 1–2 years thereafter, with more frequent screening if clinically indicated or if there is a first-degree relative with CD [23, 24]. We previously reported a CD prevalence of 7.7% in youth, while only 5.1% of this older cohort had CD. It is recognized that CD is more common in those diagnosed with type 1 diabetes aged < 5 years [25], however it is also possible that screening for CD was conducted less frequently in the young adult population in ADDN [26]. The need for a more efficient, targeted diagnostic CD approach has been advocated. In a study undertaken in the Netherlands, for example, 42% of people with type 1 diabetes who came to develop CD were not diagnosed (with CD) until 10 years following type 1 diabetes onset [10]. Ideally, Australian national guidelines that were published almost a decade ago, should be revised and broadly implemented [27]. CD screening at type 1 diabetes diagnosis, within 2–5 years thereafter and at times where there are symptoms suggestive of CD, in addition to further research to determine screening frequency beyond 5 years of diabetes duration, has been advocated [25]. Various clinical indications for CD screening beyond just type 1 diabetes have also been shared [28]; especially important considering increasing worldwide CD incidence [29].
Our findings should be interpreted with some caution. We did not examine socioeconomic characteristics or input from differing healthcare professionals. Though our study did not consider dietetic input, there was a trend towards more visits in this population. Further, we did not consider adherence to a gluten free diet which, in youth with coexisting type 1 diabetes and CD, leads to regular growth and stable BMI, without any negative effect on HbA1c and insulin requirements [15]. We also did not examine loss to contact when transitioning from paediatric to adult type 1 diabetes healthcare services, and data were not available on use of use of continuous glucose monitoring in this population. ADDN data are also predominantly derived from public diabetes clinics; therefore data from general practice or private practice were not fully captured [30]. Finally, use of a tighter age range to represent adolescents and young adults, especially when considering typical ages of rebellion and discomfort, may have yielded differing results. Nevertheless, a major strength of our research is the sample size, and volume of available HbA1c measurements and clinical data; the size and scope of our database suggests our data are reasonably representative. Moreover, data were derived from across varying geographic regions.
In conclusion, we have demonstrated that coexisting type 1 diabetes and CD is associated with a small, but statistically significantly lower HbA1c, when compared to those with type 1 diabetes alone. However, HbA1c remains above target in both groups. Findings indicate that clinicians should be aware of risk factors for higher HbA1c, namely longer type 1 diabetes duration, BP in the hypertensive range and elevated BMI.
Change history
02 September 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00592-023-02160-6
References
Taczanowska A, Schwandt A, Amed S et al (2021) Celiac disease in children with type 1 diabetes varies around the world: An international, cross-sectional study of 57,375 patients from the SWEET registry. J Diabetes 13(6):448–457
Bybrant M, Udén E, Frederiksen F et al (2021) Celiac disease can be predicted by high levels of tissue transglutaminase antibodies in children and adolescents with type 1 diabetes. Pediatr Diabetes 22:417–424
Craig M, Prinz N, Boyle CT et al (2017) Prevalence of celiac disease in 52,721 youth with type 1 diabetes: international comparison across three continents. Diabetes Care 40(8):1034–1040
Gheshlagh R, Rezaei H, Goli M et al (2020) Prevalence of celiac disease in Iranian patients with type 1 diabetes: A systematic review and meta-analysis. Indian J Gastroenterol 39(5):419–425
Singh P, Arora A, Strand TA et al (2018) Global prevalence of celiac disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol 16(6):823–836
Pham-Short A, Donaghue KC, Ambler G et al (2019) Abnormal cortical and trabecular bone in youth with type 1 diabetes and celiac disease. Diabetes Care 42(8):1489–1495
Leeds J, Hopper AD, Hadjivassiliou M et al (2011) High prevalence of microvascular complications in adults with type 1 diabetes and newly diagnosed celiac disease. Diabetes Care 34(10):2158–2163
Mollazadegan K, Kugelberg M, Montgomery SM et al (2013) A population-based study of the risk of diabetic retinopathy in patients with type 1 diabetes and celiac disease. Diabetes Care 36:316–321
Rohrer T, Wolf J, Liptay S et al (2015) Microvascular complications in childhood-onset type 1 diabetes and celiac disease: a multicenter longitudinal analysis of 56,514 patients from the German-Austrian DPV database. Diabetes Care 38(5):801–807
Bakker S, Tushuizen ME, von Blomberg ME et al (2013) Type 1 diabetes and celiac disease in adults: glycemic control and diabetic complications. Acta Diabetol 50(3):319–324
Pham-Short A, Donaghue K, Ambler G et al (2014) Early elevation of albumin excretion rate is associated with poor gluten-free diet adherence in young people with coeliac disease and diabetes. Diabet Med 31(2):208–212
Malalasekera V, Cameron F, Grixti E et al (2009) Potential reno-protective effects of a gluten-free diet in type 1 diabetes. Diabetologia 52(5):798–800
Mollazadegan K, Fored M, Lundberg S et al (2014) Risk of renal disease in patients with both type 1 diabetes and coeliac disease. Diabetologia 57(7):1339–1345
Creanza A, Lupoli R, Lembo E et al (2018) Glycemic control and microvascular complications in adults with type 1 diabetes and long-lasting treated celiac disease: A case-control study. Diabetes Res Clin Pract 143:282–287
Mozzillo E, Franceschi R, Di Candia F et al (2022) The impact of gluten-free diet on growth, metabolic control and quality of life in youth with type 1 diabetes and celiac disease: a systematic review. Diabetes Res Clin Pract 191:110032
Dovey-Pearce G, Hurrell R, May C (2005) Young adults (16–25 years) suggestions for providing developmentally appropriate diabetes services: a qualitative study. Health Soc Care Comm 13:409–419
James S, Perry L, Lowe J et al (2022) Suboptimal glycemic control in adolescents and young adults with type 1 diabetes from 2011 to 2020 across Australia and New Zealand: data from the Australasian Diabetes Data Network registry. Pediatr Diabetes 23(6):736–741
James S, Perry L, Gallagher R et al (2020) A discussion of healthcare support for adolescents and young adults with long-term conditions: current policy and practice and future opportunities. Int J Nurs Pract 26(5):e12882
Phelan H, Clapin H, Bruns L et al (2017) The Australasian Diabetes Data Network: first national audit of children and adolescents with type 1 diabetes. Med J Aust 206(3):121–125
National Institutes of Health, The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. NIH Publication No. 05–5267. Available at: https://www.nhlbi.nih.gov/health-topics/fourth-report-on-diagnosis-evaluation-treatment-high-blood-pressure-in-children-and-adolescents, Accessed 11 Aug 2022. 2005.
Centers for Disease Control and Prevention, Growth charts. Available at: http://www.cdc.gov/growthcharts/data/zscore/bmiagerev.xls, accessed 11th August 2022. 2013.
Di Meglio L, Acerini CL, Codner E et al (2018) 2018 ISPAD Clinical Practice Consensus Guidelines: Glycemic control targets and glucose monitoring for children, adolescents, and young adults with diabetes. Pediatr Diabetes 19:105–114
Kordonouri O, Klingensmith G, Knip M et al (2014) Other complications and diabetes associated conditions in children and adolescents. Pediatr Diabetes 15:270–278
Fröhlich-Reiterer E, Elbarbary NS, Simmons K et al (2022) ISPAD Clinical Practice Consensus Guidelines 2022: Other complications and associated conditions in children and adolescents with type 1 diabetes. Pediatr Diabetes 23(8):1451–1467
Pham-Short A, Donaghue KC, Ambler G et al (2015) Screening for Celiac Disease in Type 1 Diabetes: A Systematic Review. Pediatrics 136:e170
Dixit R, Lebwohl B, Ludvigsson JF et al (2014) Celiac disease is diagnosed less frequently in young adult males. Dig Dis Sci 59(7):1509–1512
Australian Type 1 Diabetes Guidelines Expert Advisory Group, National evidence-based clinical care guidelines for type 1 diabetes in children, adolescents and adults. Australian Government: Canberra. 2011.
Singh P, Singh AD, Ahuja V et al (2022) Who to screen and how to screen for celiac disease. World J Gastroenterol 28(32):4493–4507
King J, Jeong J, Underwood FE et al (2020) Incidence of celiac disease is increasing over time: a systematic review and meta-analysis. Am J Gastroenterol 115(4):507–552
Diabetes Australia, Type 1 diabetes. Available at: https://www.ndss.com.au/about-the-ndss/diabetes-facts-and-figures/diabetes-data-snapshots/, accessed 10th March 2021. 2020.
Acknowledgements
This research was conducted as part of the Australasian Diabetes Data Network (ADDN), which is supported by the Australian Type 1 Diabetes Clinical Research Network, led by the Juvenile Diabetes Research Foundation (JDRF) Australia, the recipient of Australian Government funding from the Australian Research Council (through a Special Research Initiative) and the Department of Health and Ageing. We are grateful to the children and young people with diabetes and their families who contribute to ADDN and to members of the ADDN Study Group who provided the data and reviewed the manuscript.
Australasian Diabetes Data Network (ADDN) Study Group members: Clinical Professor Geoff Ambler, The Children’s Hospital at Westmead, Sydney; Professor Dr Kym Anderson, University Hospital Geelong, Geelong; A/Prof Sof Andrikopoulos, Australian Diabetes Society; Professor Jenny Batch, Queensland Children's Hospital, Brisbane; Dr Justin Brown, Monash Children’s Hospital, Melbourne; Professor Fergus Cameron, Royal Children’s Hospital, Melbourne; Professor Peter G. Colman, The Royal Melbourne Hospital, Melbourne; A/Prof Louise Conwell, Queensland Children's Hospital, Brisbane; A/Prof Andrew Cotterill, Queensland Children's Hospital, Brisbane; Professor Jennifer Couper, Women’s and Children’s Hospital, Adelaide; A/Prof Elizabeth Davis, Perth Children’s Hospital, Perth; Dr Martin de Bock, Christchurch Hospital, New Zealand; Dr Jan Fairchild, Women’s and Children’s Hospital, Adelaide; Dr Gerry Fegan, Fiona Stanley Hospital, Perth; A/Prof Spiros Fourlanos, Royal Melbourne Hospital, Melbourne; A/Prof Sarah Glastras, Royal North Shore Hospital, Sydney; Dr Peter Goss, Team Diabetes; Dr Leonie Gray, Leonie Gray Pty Ltd. A/Prof Peter Shane Hamblin, Western Health, Melbourne; Professor Paul Hofman, University of Auckland, New Zealand; A/Prof Dianne Jane Holmes-Walker, Westmead Hospital, Sydney; A/Prof Tony Huynh, Queensland Children's Hospital, Brisbane; Sonia Isaacs, University of New South Wales, Sydney, A/Prof Craig Jefferies, Starship Children’s Health, New Zealand; Dr Stephanie Johnson, Queensland Children's Hospital, Brisbane; Clinical Professor Tim Jones, Perth Children’s Hospital, Perth; Dr Jeff Kao, Royal Children’s Hospital, Melbourne; A/Prof Bruce R King, John Hunter Children’s Hospital, Newcastle; Dr Antony Lafferty, The Canberra Hospital, Canberra; Ms Michelle Martin, Illawarra Shoalhaven Diabetes Service, Wollongong; Dr Robert McCrossin, Nambour Hospital, Nambour; Dr Kris Neville, Sydney Children’s Hospital, Sydney; Dr Mark Pascoe, Royal Hobart Hospital, Hobart; Dr Ryan Paul, Waikato Hospital, New Zealand; Dr Alexia Peña, The University of Adelaide, Adelaide; Dr Liza Phillips, The Mater Private Hospital, Brisbane; A/Prof Darrell Price, Gold Coast Hospital, Gold Coast; A/Prof Christine Rodda, University of Melbourne, Melbourne; Prof David Simmons, Campbelltown Hospital, Sydney; Professor Richard Sinnott, eResearch, University of Melbourne, Melbourne; Dr Carmel Smart, John Hunter Children’s Hospital, Newcastle; Dr Monique Stone, Northern Beaches Hospital, Sydney; A/Prof Steve Stranks, Southern Adelaide Diabetes and Endocrine Services, Adelaide; Dr Elaine Tham, Women’s and Children’s Hospital, Adelaide; Barbara Waddell, University of New South Wales, Sydney, A/Prof Glenn Ward, St. Vincent's Hospital, Melbourne; A/Prof Ben Wheeler, Dunedin School of Medicine, New Zealand; Dr Helen Woodhead, Sydney Children’s Hospital, Sydney; and Dr Anthony Zimmermann, Lyell McEwin Hospital, Elizabeth Vale.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. Maria E. Craig was supported by a NHMRC practitioner fellowship (APP1136735).
Author information
Authors and Affiliations
Consortia
Contributions
All authors contributed to the study design and the manuscript. Data were analyzed by Steven James and Maria E. Craig.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical Standard Statement
All centres had Human Research or Health and Disability Ethics Committee approval for participation in the Australasian Diabetes Data Network (ADDN); ADDN has an optout consent model. The current study was approved by the University of the Sunshine Coast Human Research Ethics Committee, Australia (reference: E19113).
Informed Consent
All of the young people have provided consent for their data to be used in the Australasian Diabetes Data Network registry.
Additional information
Managed By Massimo Federici.
The details of the “ADDN Study Group” are listed in the Acknowledgement section.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Study group name added to author group and also few text corrections updated.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
James, S., Perry, L., Lowe, J. et al. Coexisiting type 1 diabetes and celiac disease is associated with lower Hba1c when compared to type 1 diabetes alone: data from the Australasian Diabetes Data Network (ADDN) registry. Acta Diabetol 60, 1471–1477 (2023). https://doi.org/10.1007/s00592-023-02113-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-023-02113-z