Abstract
Aim
To evaluate the accuracy of DiaBetter, DiaRem, Ad-DiaRem and 5y-Ad-DiaRem scores’ at predicting T2D remission 10 or more years after surgery.
Methods
Patients with obesity and T2D (n = 126) submitted to RYGB with 10 or more years of follow-up. It was a unicentric trial. Pre-operative anthropometric and clinical data was retrieved to calculate DiaRem, DiaBetter, Ad-DiaRem and 5y-Ad-DiaRem scores, while a hospital visit was conducted to assess current diabetes status. The area under the receiver operating characteristic (AUROC) curve was calculated as estimate of the scores’ accuracy to predict long-term T2D remission.
Results
Among the entire cohort (n = 126), 70 subjects (55.6%) achieved and maintained T2D remission 10 or more years after RYGB. The 5y-Ad-DiaRem score was the one that depicted the highest discriminative power (AUROC = 0.838) to predict long-term T2D remission when compared to DiaBetter (AUROC = 0.735), DiaRem (AUROC = 0.721) and Ad-DiaRem (AUROC = 0.720).
Conclusion
The score with highest accuracy to predict long-term T2D remission after RYGB surgery was the 5y-Ad-DiaRem. Yet, the available scores accuracy to predict T2D remission in the long term is still suboptimal, highlighting the unmet need for a better scoring system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bariatric surgery is the most effective treatment for severe obesity [1]. Roux-en-Y Gastric Bypass (RYGB) is a widely performed bariatric surgery procedure with a very favourable benefit-to-risk ratio. The vast majority of patients submitted to RYGB experience substantial and sustained weight loss [2], as well as, significant improvement or resolution of obesity-associated comorbidities, such as Type 2 Diabetes (T2D) and metabolic syndrome [3,4,5,6]. Nevertheless, within the first two years after surgery 5–15% of the patients fail to achieve clinically relevant weight loss or diabetes remission [7]. A percentage that tends to increase along the timespan after surgery with an estimated long-term failure rate of 20–35% [8, 9]. This figure was corroborated by our previous study that evaluated the weight loss and comorbidities remission rate 10 or more years after RYGB, which allowed to demonstrate that less than one-third of the patients did not achieve the weight loss goal and only 54.2% of patients achieved T2D remission [10].
Several clinical and biochemical patient features have been so far identified as predictors of T2D remission. Major determinants include the magnitude of preserved pancreatic function and peripheral insulin resistance reduction [11]. Thereby, preoperative features such as advanced age, higher body mass index (BMI), longer T2D duration, greater glycated haemoglobin (HbA1c), higher fasting glucose levels, lower C-peptide levels and use of insulin therapy tend to be associated with lower probability of T2D remission after bariatric surgery [12, 13]. In particular, a previous study found that a preoperative HbA1c < 7.1% have a sensitivity of 85% to predict T2D remission at 1 year after surgery [14]. Additionally, a greater total weight loss (%TWL) after surgery tends to be a positive predictor of T2D remission [15].
Based on the above mentioned determinants of T2D remission, several predictive models have been devised, such as DiaBetter [16], DiaRem [17], Ad-DiaRem [18] and 5y-Ad-DiaRem [19]. These mathematical models were demonstrated to predict T2D remission in the short-term, with an accuracy that ranges between 75 and 90% for RYBG [13, 16, 18, 20, 21]. Additionally, the 5y-Ad-DiaRem score used to predict medium-term T2D remission, includes not only preoperative determinants but also weight loss 1-year after surgery [19]. Anyhow, the accuracy of the above mentioned scores to predict T2D remission tends to decrease along follow-up time, to under 75% at medium term, i.e. 5 or more years after surgery [22, 23], while the accuracy of these models at predicting long-term T2D remission has not been reported.
Thus, the aim of the present study was to evaluate the accuracy of DiaBetter, DiaRem, Ad-DiaRem and 5y-Ad-DiaRem scores at predicting long-term T2D remission, i.e., 10 or more years after RYGB surgery.
Methods
Patient’s and methods
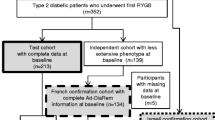
This was a single centre retrospective observational cohort study. Data from patients with obesity (BMI ≥ 35 kg/m2) submitted to RYGB, between January of 2003 and December of 2009 for obesity treatment, who completed 10 or more years of follow-up after surgery, were retrieved for analysis. From the initial cohort of 281 patients, 83 had a pre-operative diagnosis of diabetes [10]. This initial cohort was later expanded by 71 additional patients with type 2 diabetes submitted to RYGB between January of 2010 and December of 2011, who at the time of the initial cohort recruitment, had not yet completed 10 years of follow-up after RYGB. Thereby, 154 patients were pre-selected to be enrolled in this study. Patients with a previous bariatric intervention who underwent revisional surgery, who had a diagnosis of diabetes other than type 2, such as type 1 diabetes or Latent Autoimmune Diabetes of the Adult (LADA) or did not have enough data available to calculate at least one of the four prediction scores, were excluded from the study. Consequently, 126 patients were included in this study for statistical analysis (Fig. 1).
Preoperative clinical evaluation consisted of a full medical history, physical examination and fasting blood analysis. The parameters evaluated were age, body weight, BMI, comorbidities, ongoing drugs, fasting blood glucose and HbA1c.
All patients underwent bariatric surgery at a single centre by the same team of bariatric surgeons. The surgical procedure consisted in a standard RYGB procedure with a constant alimentary limb length (120 cm) and a variable biliopancreatic limb length (50–200 cm) [24].
After surgery, patients were followed up by a multidisciplinary team (bariatric surgeon, endocrinologist, psychologist and registered dietitian) attending regular outpatient hospital visits for the first three years after surgery, and thereafter by the general practitioner. Routine hospital visits included sequential revaluation of body weight and fasting blood analysis that included the measurements of glucose and HbA1c.
Patients were considered as being in T2D remission, at 1-year and 10 or more years after surgery, if HbA1c was under 6.5% without use of any antidiabetic drug and as having diabetes relapse whenever HbA1c was above 6.5% with use of any antidiabetic drug after experiencing a period of remission [25].
Preoperative anthropometric and clinical data, including age, HbA1c, T2D duration and the number and type of antidiabetic drugs, were used to calculate DiaBetter [16], DiaRem [17], Ad-DiaRem [18] scores. Among the 3 diabetes remission prediction scores, DiaRem is the only one that does not takes into consideration T2D duration, while Ad-DiaRem considers not only T2D duration but also the number of antidiabetic drugs used, and DiaBetter score is the most simple score to calculate, as it only considers 3 parameters (HbA1c, T2D duration and the type of antidiabetic medication) weighted on a scale between 0 and 3. For the 5y-Ad-DiaRem [19] score, calculations required a combination of preoperative and postoperative 1-year parameters, namely number of antidiabetic drugs, fasting blood glucose, body weight lost and 1-year T2D remission status.
Calculations and statistical analysis
Anthropometric data were used to calculate Body Mass Index (BMI, calculated as, [weight (Kg) ÷ height2 (m2)]), percentage of total weight loss (% TWL, calculated as [[(preoperative weight–postoperative weight) ÷ (preoperative weight)] × 100]) and percentage of excess weight loss (% EWL, calculated as [[(preoperative weight–postoperative weight) ÷ (preoperative weight–(25 × height2 (m2)))] × 100]).
Categorical variables are expressed as number of cases and percentage (%), and the quantitative variables are expressed as mean and standard deviation. Categorical variables were analyzed by the χ2 test. To compare three or more independent experimental groups, we used the one-way ANOVA for normally distributed variables and the Kruskal–Wallis for variables that did not present a normal distribution.
The diagnostic accuracy of these scores for predicting long-term T2D remission was evaluated using the receiver operating characteristic (ROC) curve. ROC curves were used to compare the accuracy of prediction models (DiaRem, DiaBetter, Ad-DiaRem and 5y-Ad-DiaRem) between the different T2D remission groups: patients with T2D remission (10y-DR), patients that never experienced T2D remission during the 10 years (10y-NDR), patients that experienced T2D remission but relapsed (10y-Relapse) and patients with T2D at 10 years (non-remission group) that merges the 2 previous groups. In a ROC curve, the true positive rate (sensitivity) is represented as a function of the false positive rate (1-specificity) for different cut-off points of a parameter. The area under the ROC curve (AUROC) was used to measure how accurate is a score at predicting T2D remission. Based on the AUROC, the test can be considered excellent between 0.90 and 1.00; good between 0.80 and 0.90; fair between 0.70 and 0.80; and poor between 0.60 and 0.70; and failed for values below 0.60.
A p-value < 0.05 was considered statistically significant. All statistical analyses were performed with the aid of the GraphPad Prism software version 8.0 and IBM SPSS Statistics version 27, both for Windows.
Results
The long-term outcomes of a cohort of 126 patients with obesity and T2D submitted to laparoscopic RYBG at a single centre were assessed. Of these patients, 80.2% experienced T2D remission 1 year after surgery, whereas the rate of T2D remission decreased to 55.6% (n = 70) at 10 years after RYGB. Therefore, 33 patients (32.7%) with T2D remission in the first year after surgery (1y-DR), were found to have relapsed 10 years after RYGB (Fig. 2).
Evolution of T2D status in 126 patients of the cohort study, throughout the follow-up, 1 and 10 years after RYGB. T2D remission was considered if HbA1c was below 6.5%, in the absence of any antidiabetic drug. Abbreviations: 1y-DR – Diabetes remission in the first year after RYGB; 1y-NDR – No diabetes remission in the first year after RYGB; 10y-DR – Diabetes remission group at 10 or more years after RYGB; 10y-Relapse – Relapse group at 10 or more years after RYGB; 10-NDR – Never diabetes remission group at 10 or more years after RYGB
Afterwards, clinical and biochemical parameters of patients with different trajectories: 10y-DR, 10y-Relapse and 10y-NDR were compared (Table 1).
These three groups are statistically different in baseline pre-operative T2D related characteristics and in the percentage of body weight loss two years after RYGB. Patients that experienced long-term T2D remission presented a statistically significant shorter duration of diabetes, lower preoperative fasting glucose and HbA1c, and less use of insulin therapy, when compared with other two groups. Although the 10y-DR subgroup had a greater BMI at baseline, patients experienced greater weight loss after bariatric surgery, particularly within the first year after RYGB (%TWL: 33.8 ± 6.8, 30.0 ± 5.3 and 29.7 ± 8.8 (p = 0.007), for 10y-DR, 10y-Relapse and 10-NDR groups, respectively). Differences in EWL were statistically significant between the three groups at the second year after surgery (%EWL: 82.3 ± 20.1, 71.5 ± 15.8 and 71.0 ± 22.4 (p = 0.013), for 10y-DR, 10y-Relapse and 10-NDR groups, respectively) (Table 1).
There was a statistically significant difference in the mean AUROC values of T2D remission prediction scores, with an increasing order from 10y-DR to 10y-Relapse and 10y-NDR (p < 0.001), respectively. The long-term accuracy of T2D remission prediction scores is shown in Fig. 3. When comparing the remission group with the non-remission group (10y-Relapse and 10-NDR), the AUROC was 0.721 for DiaRem, 0.735 for DiaBetter, 0.720 for Ad-DiaRem and 0.838 for 5y-Ad-DiaRem (Fig. 3a). These values increased to 0.787 for DiaRem, 0.769, for DiaBetter, 0.791 for Ad-DiaRem and 0.964 for 5y-Ad-DiaRem, only when 10y-DR and 10y-NDR when compared (Fig. 3b).
Comparison of the accuracy of prediction models (DiaRem, DiaBetter, Ad-DiaRem and 5y-Ad-DiaRem) in the study population using receiver operating characteristic. (A: Comparison between remission and non-remission group (10y-Relapse + 10y-NDR) of T2D at 10 or more years; B: Comparison between 10y-DR and 10y-NDR groups; C: Comparison between 10y-DR and 10y-Relapse groups). The orange line serves as the reference line
Discussion
Several prediction models can be used to predict the probability of T2D remission in the short term after bariatric surgery. However, the accuracy of these scores at predicting long-term T2D remission was not yet reported. Therefore, the primary aim of the present study was to evaluate the accuracy of pre-stablished prediction scores at predicting long-termT2D remission, i.e. 10 or more years after RYGB.
In the medium and long-term after bariatric surgery, patients tend to experience some degree of weight regain and the percentage of obesity-associated comorbidities resolution decreases, with a reduction in T2D remission rates between the first two years and 10 years after surgery ranging from 28.4 to 32.0% [5, 6]. The present study corroborates these findings, since a T2D remission rate of 80.2% was observed in the first year after RYGB, while the percentage of T2D remission at 10 years after surgery was 55.6%, representing a reduction of 30.7%, of subjects that achieved and maintained T2D remission. A 51.4% reduction in the rates of T2D remission between the second year (72%) and tenth year (35%) was previously reported in the Swedish Obese Subjects (SOS) study [23]. However, this data may not be comparable to the herein study findings, since among the patients included in the SOS study only 17.9% had been submitted to gastric bypass. Furthermore, it is important to highlight the heterogeneity in the diabetes remission criteria adopted by the different studies and across times that hinders comparisons of diabetes remission rates between studies, which should always be carried out with utmost caution [11, 25].
In the herein study, thirty-six patients (28.6%) experienced T2D remission during the follow-up, but had a relapse 10 or more years after RYBG. Similar relapse rates were observed by other authors [26].
The long-term T2D remission in this study was associated with a shorter duration of diabetes, lower preoperative fasting glucose and HbA1c, and less use of insulin therapy before surgery, which are known to be associated with a greater β-cell pancreatic function reserve. Therefore, these results support previous findings that T2D duration and C-peptide levels, are among the most powerful predictors of diabetes remission [27,28,29]. The impact of this biochemical markers is so relevant that C-peptide was included in another diabetes remission prediction score, the ABCD score [30]. However, its widespread use in the clinical setting can be hindered by the fact that C-peptide measurement is not widely available in many routine laboratories, as occurs at our centre. In the absence of C-peptide data, the pancreatic function reserve can still be inferred by the degree of glycaemic control and the need of insulin therapy for glycaemic control prior to bariatric surgery.
T2D outcomes over the long-term after RYBG, beyond pre-operative predictors, is mainly dependent on weight loss within the first year after surgery and weight maintenance over time. Thus, the importance of dietary and lifestyle interventions during the first year after surgery in order to maximize the potential weight loss in order to achieve long-term T2D remission [31], must be stressed and should be a take home message for the clinic. In our study, patients with long-term T2D remission had a TWL in the first year after RYBG 13.0% greater than in patients who presented T2D diagnosis criteria 10 or more years after surgery, and an EWL, at 2 years after surgery, 15.3% greater than long-term T2D no-remission patients.
Comparing the different scores, the 5y-Ad-DiaRem was the one with the highest accuracy at predicting T2D remission at 10 or more years after surgery. Of notice and contrarily to other scores, the 5y-Ad-DiaRem score includes the weight loss and the glycaemic control in the first year after bariatric surgery. Debédat and colleagues when presenting this score defended that pre-operative glycaemic control was more impactful to discriminate patients with T2D remission and without T2D remission 5 years after RYGB. On the other hand, the authors also demonstrated that weight loss in the first year had more influence on the risk of T2D relapse at the end of the same follow-up period [19]. Our current study corroborates the previous findings, given that when comparing patients who did not meet the diagnosis criteria for T2D 10 or more years after surgery with those who did, the 5y-Ad-DiaRem score had a greater accuracy in terms of discriminative power than the other studied scores that only presented a fair accuracy, with values below 0.74.
The accuracy of T2D remission prediction scores increases when the comparison is restricted to 10y-DR and 10y-NDR patients, with the greatest increase for 5y-Ad-DiaRem (AUROC = 0.964). The excellent discrimination power of 5y-Ad-DiaRem, supports the importance of weight loss and the glycaemic control in first year after surgery for the long-term T2D outcomes.
Preoperative prediction of T2D remission can be also useful to address and manage patient expectations on the outcomes of bariatric interventions. Nonetheless, given that a greater weight loss in the first few years after surgery is associated with higher probability of long-term T2D remission, medical and lifestyle interventions in the early post-operative period should be optimized in order to achieve the greatest possible weight loss.
Regardless of the fact that part of the patients never achieve diabetes remission, or experience only a transient remission, there are still significant long-term benefits, which can be translated by a reduction in the needs of antidiabetic drugs, a reduction of Hb1Ac values and a decrease of morbidity and mortality associated with T2D, benefits that the scores do not have the ability to predict [32]. In this study, 41.5% of the patients with BMI > 45 kg/m2 did not achieved long-term T2D remission. Therefore, an alternativesurgical procedure, associated with higher rates of diabetes remission and TWL, or an adjuvant medical treatment, after surgery, with antidiabetic drugs, such as metformin, should have been considered.
This study presents some limitations that must be taken into consideration. This is an observational single centre retrospective study, which can confer some risk of bias and limit the size of data available for analysis. The small number of patients with T2D relapse and never remission at 10 years after RYBG can overestimate the scores’ accuracy, whereby there is a need to validate our findings in different and larger patient cohorts.
In conclusion, RYGB is associated with a high rate of long-term T2D remission that can be estimated by the use of prediction scores. Among, DiaRem, Ad-DiaRem, 5y-Ad-DiaRem and DiaBetter scores, the 5y-Ad-DiaRem was the one that demonstrated to have the highest accuracy to predict long-term T2D remission after RYGB surgery. Nevertheless, the accuracy of the available scores to predict T2D remission in the long term is considerably decreased over the timespan after surgery. Thus, a better scoring system for predicting long-term T2D remission after bariatric surgery is still needed.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Sjoholm K, Svensson PA, Taube M et al (2020) Evaluation of prediction models for type 2 diabetes relapse after post-bariatric surgery remission: a post hoc analysis of 15-year follow-up data from the swedish obese subjects (SOS) study. Obes Surg 30(10):3955–3960. https://doi.org/10.1007/s11695-020-04763-2
O’Brien PE, Hindle A, Brennan L et al (2019) Long-term outcomes after bariatric surgery: a systematic review and meta-analysis of weight loss at 10 or more years for all bariatric procedures and a single-centre review of 20-year outcomes after adjustable gastric banding. Obes Surg 29(1):3–14. https://doi.org/10.1007/s11695-018-3525-0
Sjostrom L, Lindroos AK, Peltonen M et al (2004) Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 351(26):2683–2693. https://doi.org/10.1056/NEJMoa035622
Buchwald H, Estok R, Fahrbach K et al (2009) Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 122(3):248-256.e5. https://doi.org/10.1016/j.amjmed.2008.09.041
Adams TD, Davidson LE, Litwin SE et al (2017) Weight and metabolic outcomes 12 years after gastric bypass. N Engl J Med 377(12):1143–1155. https://doi.org/10.1056/NEJMoa1700459
Moriconi D, Manca ML, Anselmino M et al (2021) Predictors of type 2 diabetes relapse after Roux-en-Y gastric bypass: a ten-year follow-up study. Diabetes Metab. https://doi.org/10.1016/j.diabet.2021.101282
Irani K, Youn HA, Ren-Fielding CJ, Fielding GA, Kurian M (2011) Midterm results for gastric banding as salvage procedure for patients with weight loss failure after Roux-en-Y gastric bypass. Surg Obes Relat Dis 7(2):219–224. https://doi.org/10.1016/j.soard.2010.09.024
Christou NV, Look D, Maclean LD (2006) Weight gain after short- and long-limb gastric bypass in patients followed for longer than 10 years. Ann Surg 244(5):734–740. https://doi.org/10.1097/01.sla.0000217592.04061.d5
Arterburn DE, Bogart A, Sherwood NE et al (2013) A multisite study of long-term remission and relapse of type 2 diabetes mellitus following gastric bypass. Obes Surg 23(1):93–102. https://doi.org/10.1007/s11695-012-0802-1
Guimaraes M, Osorio C, Silva D et al (2021) How sustained is Roux-en-Y gastric bypass long-term efficacy? : Roux-en-Y gastric bypass efficacy. Obes Surg 31(8):3623–3629. https://doi.org/10.1007/s11695-021-05458-y
Park JY (2018) Prediction of type 2 diabetes remission after bariatric or metabolic surgery. J Obes Metab Syndr 27(4):213–222. https://doi.org/10.7570/jomes.2018.27.4.213
Wang GF, Yan YX, Xu N et al (2015) Predictive factors of type 2 diabetes mellitus remission following bariatric surgery: a meta-analysis. Obes Surg 25(2):199–208. https://doi.org/10.1007/s11695-014-1391-y
Cotillard A, Poitou C, Duchateau-Nguyen G et al (2015) Type 2 diabetes remission after gastric bypass: what is the best prediction tool for clinicians? Obes Surg 25(7):1128–1132. https://doi.org/10.1007/s11695-014-1511-8
Robert M, Ferrand-Gaillard C, Disse E et al (2013) Predictive factors of type 2 diabetes remission 1 year after bariatric surgery: impact of surgical techniques. Obes Surg 23(6):770–775. https://doi.org/10.1007/s11695-013-0868-4
Park JY, Kim YJ (2016) Prediction of diabetes remission in morbidly obese patients after Roux-en-Y gastric bypass. Obes Surg 26(4):749–756. https://doi.org/10.1007/s11695-015-1823-3
Pucci A, Tymoszuk U, Cheung WH et al (2018) Type 2 diabetes remission 2 years post Roux-en-Y gastric bypass and sleeve gastrectomy: the role of the weight loss and comparison of DiaRem and DiaBetter scores. Diabet Med 35(3):360–367. https://doi.org/10.1111/dme.13532
Still CD, Wood GC, Benotti P et al (2014) Preoperative prediction of type 2 diabetes remission after Roux-en-Y gastric bypass surgery: a retrospective cohort study. Lancet Diabetes Endocrinol 2(1):38–45. https://doi.org/10.1016/S2213-8587(13)70070-6
Aron-Wisnewsky J, Sokolovska N, Liu Y et al (2017) The advanced-DiaRem score improves prediction of diabetes remission 1 year post-Roux-en-Y gastric bypass. Diabetologia 60(10):1892–1902. https://doi.org/10.1007/s00125-017-4371-7
Debedat J, Sokolovska N, Coupaye M et al (2018) Long-term relapse of Type 2 diabetes after Roux-en-Y gastric bypass: prediction and clinical relevance. Diabetes Care 41(10):2086–2095. https://doi.org/10.2337/dc18-0567
Karpinska IA, Choma J, Wysocki M et al (2021) External validation of predictive scores for diabetes remission after metabolic surgery. Langenbecks Arch Surg. https://doi.org/10.1007/s00423-021-02260-3
Kam H, Tu Y, Pan J et al (2020) Comparison of four risk prediction models for diabetes remission after Roux-en-Y gastric bypass surgery in obese Chinese patients with type 2 diabetes mellitus. Obes Surg 30(6):2147–2157. https://doi.org/10.1007/s11695-019-04371-9
Dicker D, Golan R, Aron-Wisnewsky J, Zucker JD, Sokolowska N, Comaneshter DS et al (2019) Prediction of long-term diabetes remission after RYGB, sleeve gastrectomy, and adjustable gastric banding using DiaRem and advanced- DiaRem scores. Obes Surg 29(3):796–804. https://doi.org/10.1007/s11695-018-3583-3
Sjoholm K, Carlsson LMS, Taube M, le Roux CW, Svensson PA, Peltonen M (2020) Comparison of preoperative remission scores and diabetes duration alone as predictors of durable type 2 diabetes remission and risk of diabetes complications after bariatric surgery: a post Hoc analysis of participants from the swedish obese subjects study. Diabetes Care 43(11):2804–2811. https://doi.org/10.2337/dc20-0157
Nora M, Morais T, Almeida R, Guimaraes M, Monteiro MP (2017) Should Roux-en-Y gastric bypass biliopancreatic limb length be tailored to achieve improved diabetes outcomes? Medicine (Baltimore). 96(48):e8859. https://doi.org/10.1097/MD.0000000000008859
Riddle MC, Cefalu WT, Evans PH, Gerstein HC, Nauck MA, Oh WK et al (2021) Consensus report: definition and interpretation of remission in type 2 diabetes. Diabetologia 64(11):2359–2366. https://doi.org/10.1007/s00125-021-05542-z
Aminian A, Vidal J, Salminen P, Still CD, Nor Hanipah Z, Sharma G et al (2020) Late relapse of diabetes after bariatric surgery: not rare, but not a failure. Diabetes Care 43(3):534–540. https://doi.org/10.2337/dc19-1057
Fultang J, Chinaka U, Rankin J, Bakhshi A, Ali A (2021) Preoperative bariatric surgery predictors of type 2 diabetes remission. J Obes Metab Syndr 30(2):104–114. https://doi.org/10.7570/jomes20084
Brethauer SA, Aminian A, Romero-Talamás H, Batayyah E, Mackey J, Kennedy L, Kashyap SR, Kirwan JP, Rogula T, Kroh M, Chand B, Schauer PR (2013) Can diabetes be surgically cured? Long-term metabolic effects of bariatric surgery in obese patients with type 2 diabetes mellitus. Annal Surg 258(4):628–637. https://doi.org/10.1097/SLA.0b013e3182a5034b
Aarts EO, Janssen J, Janssen IM, Berends FJ, Telting D, de Boer H (2013) Preoperative fasting plasma C-peptide level may help to predict diabetes outcome after gastric bypass surgery. Obes Surg 23(7):867–873. https://doi.org/10.1007/s11695-013-0872-8
Lee WJ, Hur KY, Lakadawala M, Kasama K, Wong SK, Chen SC et al (2013) Predicting success of metabolic surgery: age, body mass index, C-peptide, and duration score. Surg Obes Relat Dis 9(3):379–384. https://doi.org/10.1016/j.soard.2012.07.015
Belligoli A, Bettini S, Segato G, Busetto L (2020) Predicting responses to bariatric and metabolic surgery. Curr Obes Rep 9(3):373–379. https://doi.org/10.1007/s13679-020-00390-1
Tsilingiris D, Koliaki C, Kokkinos A (2019) Remission of type 2 diabetes mellitus after bariatric surgery: fact or fiction? Int J Environ Res Publ Health 16(17):3171. https://doi.org/10.3390/ijerph16173171
Acknowledgements
Authors would like to thank Sara Andrade from the Clinical and Experimental Endocrinology, Department of Anatomy, Unit for Multidisciplinary Research in Biomedicine, Abel Salazar Institute of Biomedical Sciences, University of Porto, Porto, Portugal, for outstanding technical assistance with data.
Funding
Open access funding provided by FCT|FCCN (b-on). This study was funded by the Foundation for Science and Technology (FCT) through the following funds: PTDC/MECMET/32151/2017, UIDB/00215/2020, UIDP/00215/2020, LA/P/0064/2020 and PTDC/MEC-CIR/3615/2021, and by a grant attributed by the Grupo de Estudos de Investigação Fundamental e Translacional (GIFT) – Sociedade Portuguesa de Diabetologia (SPD), in 2022.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
This study was approved by the institutional ethical review board and all patients signed an informed consent document before conducting any study assessment and enrolment.
Ethical approval
The study was approved by the institutional ethical review board (approval number: CA-149/2020-0t_MP/AC). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Managed By Riccardo C. Bonadonna.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cardoso, S., Pereira, S.S., Almeida, R.F. et al. Accuracy of prediction models for long-term type 2 diabetes remission after gastric bypass. Acta Diabetol 60, 1019–1026 (2023). https://doi.org/10.1007/s00592-023-02092-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-023-02092-1