Abstract
Background
CD4 + T helper (Th)22 cells play a regulatory role in autoimmune diseases such as type 1 diabetes mellitus. The Th22-related cytokine interleukin (IL)-22, the expression of which is increased in diabetes mellitus (DM), can act as a neurotrophic factor to protect neurons from apoptosis. Paradoxically, neuronal apoptosis and learning and memory decline occur in DM. In this study, we investigated the relationship between IL-22 and its receptors IL-22Rα1 and IL-22 binding protein (IL-22BP, a soluble inhibitor of IL-22) in diabetic encephalopathy (DE) and the effects of IL-22 on hippocampal neurons, learning and memory.
Methods
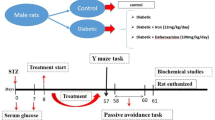
A C57BL/6 mouse model of diabetes was constructed by intraperitoneal injection of streptozotocin. The mice were randomly divided into 4 groups: the control group, diabetes group, diabetes + recombinantIL-22 (rIL-22) group and diabetes + IL-22BP group. The Morris water maze test was used to evaluate learning and memory, the expression of IL-22 was measured by ELISA, and Evans Blue staining was used to evaluate blood–brain barrier permeability. Quantitative reverse transcription polymerase chain reaction (RT-qPCR) was used to measure the mRNA expression of IL-22 and IL-22Rα1 in the hippocampus. The morphology and number of hippocampal neurons were assessed by Nissl staining, and TUNEL staining was used to detect hippocampal neuronal apoptosis. Immunofluorescence was used to analyze IL-22Rα1 expression and localization in hippocampus, and Western blotting was used to evaluate the expression of IL-22, IL-22Rα1, IL-22BP, and the apoptosis related proteins Caspase-3 and C-caspase-3.
Results
Compared with those in the control group, mice in the diabetes group showed cognitive decline; apoptosis of hippocampal neurons; increased expression of hippocampal Caspase-3, C-Caspase-3, IL-22, IL-22Rα1, and IL-22BP; and a decreased IL-22/IL-22BP ratio. Learning and memory were improved, neuronal apoptosis was attenuated, IL-22Rα1 expression and the IL-22/IL-22BP ratio were increased, and caspase-3 and C-caspase-3 expression was decreased in the rIL-22-treated group compared with the diabetes group. IL-22BP treatment aggravated diabetic cognitive dysfunction and pathological alterations in the hippocampus, decreased the IL-22/IL-22BP ratio, and increased the expression of caspase-3 and C-caspase-3 in mice with diabetes.
Conclusion
A decrease in the IL-22/IL-22BP ratio plays an important role in diabetic cognitive dysfunction, and rIL-22 can effectively alleviate DE. Herein, we shed light on the interaction between IL-22 and IL-22BP as therapeutic targets for DM.






Similar content being viewed by others
References
Bogush M, Heldt NA, Persidsky Y (2017) Blood brain barrier injury in diabetes: unrecognized effects on brain and cognition. J Neuroimmune Pharmacol 12(4):593–601
Wu H, Norton V, Cui K et al (2022) Diabetes and its cardiovascular complications: comprehensive network and systematic analyses. Front Cardiovasc Med 9:841928
Glovaci D, Fan W, Wong ND (2019) Epidemiology of diabetes mellitus and cardiovascular disease. Curr Cardiol Rep 21(4):21
Cheon SY, Song J (2021) The association between hepatic encephalopathy and diabetic encephalopathy: the brain-liver axis. Int J Mol Sci 22(1):463
Ho N, Sommers MS, Lucki I (2013) Effects of diabetes on hippocampal neurogenesis: links to cognition and depression. Neurosci Biobehav Rev 37(8):1346–1362
Kang X, Li C, Xie Y et al (2021) Hippocampal ornithine decarboxylase/spermidine pathway mediates H(2)S-alleviated cognitive impairment in diabetic rats: involving enhancment of hippocampal autophagic flux. J Adv Res 27:31–40
Shikata K, Makino H (2001) Role of macrophages in the pathogenesis of diabetic nephropathy. Contrib Nephrol 134:46–54
McNay EC, Pearson-Leary J (2020) GluT4: a central player in hippocampal memory and brain insulin resistance. Exp Neurol 323:113076
Mizuki N, Meguro A, Ota M et al (2010) Genome-wide association studies identify IL23R-IL12RB2 and IL10 as Behçet’s disease susceptibility loci. Nat Genet 42(8):703–6
Raphael I, Joern RR, Forsthuber TG (2020) Memory CD4(+) T cells in immunity and autoimmune diseases. Cells 9(3):531
Kahn SE, Hull RL, Utzschneider KM (2006) Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444(7121):840–846
Zhu X, Zhu J (2020) CD4 T helper cell subsets and related human immunological disorders. Int J Mol Sci 21(21):8011
Pan HF, Li XP, Zheng SG, Ye DQ (2013) Emerging role of interleukin-22 in autoimmune diseases. Cytokine Growth Factor Rev 24(1):51–7
Akbari H, Ghardashi M, Soleimani A, Mohammadi H, Nikoueinejad H (2018) T helper 22 pathway evaluation in type 1 diabetes and its complications. Iran J Allergy Asthma Immunol 17(3):258–264
Wu CC, Chen JS, Lu KC (2010) Aberrant cytokines/chemokines production correlate with proteinuria in patients with overt diabetic nephropathy. Clin Chim Acta 411(9–10):700–4
Wolk K, Witte E, Witte K, Warszawska K, Sabat R (2010) Biology of interleukin-22. Semin Immunopathol 32(1):17–31
Kotenko SV, Izotova LS, Mirochnitchenko OV et al (2001) Identification, cloning, and characterization of a novel soluble receptor that binds IL-22 and neutralizes its activity. J Immunol 166(12):7096–103
de Brito R, do Carmo RF, Silva BM et al (2022) Liver expression of IL-22, IL-22R1 and IL-22BP in patients with chronic hepatitis C with different fibrosis stages. Cytokine 150:155784
Brooks-Worrell BM, Juneja R, Minokadeh A et al (1999) Cellular immune responses to human islet proteins in antibody-positive type 2 diabetic patients. Diabetes 48(5):983–988
Guendel F, Kofoed-Branzk M, Gronke K et al (2020) Group 3 innate lymphoid cells program a distinct subset of il-22bp-producing dendritic cells demarcating solitary intestinal lymphoid tissues. Immunity 53(5):1015-1032.e8
Dudakov JA, Hanash AM, van den Brink MR (2015) Interleukin-22: immunobiology and pathology. Annu Rev Immunol 33:747–785
Weidenbusch M, Rodler S, Anders HJ (2015) Interleukin-22 in kidney injury and regeneration. Am J Physiol Ren Physiol 308(10):F1041–F1046
Shi L, Ji Q, Liu L et al (2020) IL-22 produced by Th22 cells aggravates atherosclerosis development in ApoE(-/-) mice by enhancing DC-induced Th17 cell proliferation. J Cell Mol Med 24(5):3064–3078
Sima AA (2010) Encephalopathies: the emerging diabetic complications. Acta Diabetol 47(4):279–293
Carranza-Naval MJ, Vargas-Soria M, Hierro-Bujalance C et al (2021) Alzheimer’s disease and diabetes: role of diet, microbiota and inflammation in preclinical models. Biomolecules 11(2):262
Yin Q, Ma J, Han X et al (2021) Spatiotemporal variations of vascular endothelial growth factor in the brain of diabetic cognitive impairment. Pharmacol Res 163:105234
Bhusal A, Lee WH, Suk K (2021) Lipocalin-2 in diabetic complications of the nervous system: physiology, pathology, and beyond. Front Physiol 12:638112
Spinelli M, Fusco S, Grassi C (2020) Brain insulin resistance impairs hippocampal plasticity. Vitam Horm 114:281–306
Kalantarian G, Ziamajidi N, Abbasalipourkabir R, et al (2019) Effect of insulin-loaded trimethyl chitosan nanoparticles on genes expression in the hippocampus of diabetic rats. J Basic Clin Physiol Pharmacol 31(2)
Kahya MC, Nazıroğlu M, Övey IS (2017) Modulation of diabetes-induced oxidative stress, apoptosis, and Ca(2+) entry through trpm2 and trpv1 channels in dorsal root ganglion and hippocampus of diabetic rats by melatonin and selenium. Mol Neurobiol 54(3):2345–2360
Trujillo-Estrada L, Nguyen C, da Cunha C et al (2019) Tau underlies synaptic and cognitive deficits for type 1, but not type 2 diabetes mouse models. Aging Cell 18(3):e12919
Zhou X, Wang S, Ding X et al (2017) Zeaxanthin improves diabetes-induced cognitive deficit in rats through activiting PI3K/AKT signaling pathway. Brain Res Bull 132:190–198
Yang W, Liu Y, Xu QQ, Xian YF, Lin ZX (2020) Sulforaphene ameliorates neuroinflammation and hyperphosphorylated tau protein via regulating the PI3K/Akt/GSK-3β pathway in experimental models of Alzheimer’s disease. Oxid Med Cell Longev 2020:4754195
Shruthi S, Mohan V, Amutha A, Aravindhan V (2016) Increased serum levels of novel T cell cytokines IL-33, IL-9 and IL-17 in subjects with type-1 diabetes. Cytokine 86:6–9
Xu T, Liu J, Li XR et al (2021) The mTOR/NF-κB pathway mediates neuroinflammation and synaptic plasticity in diabetic encephalopathy. Mol Neurobiol 58(8):3848–3862
Ma X, Song M, Yan Y et al (2021) Albiflorin alleviates cognitive dysfunction in STZ-induced rats. Aging (Albany NY) 13(14):18287–18297
Zeinivand M, Nahavandi A, Zare M (2020) Deferoxamine regulates neuroinflammation and oxidative stress in rats with diabetes-induced cognitive dysfunction. Inflammopharmacology 28(2):575–583
Pugazhenthi S, Qin L, Reddy PH (2017) Common neurodegenerative pathways in obesity, diabetes, and Alzheimer’s disease. Biochim Biophys Acta Mol Basis Dis 1863(5):1037–1045
Rom S, Zuluaga-Ramirez V, Gajghate S et al (2019) Hyperglycemia-driven neuroinflammation compromises BBB leading to memory loss in both diabetes mellitus (DM) type 1 and type 2 mouse models. Mol Neurobiol 56(3):1883–1896
Ruterbusch M, Pruner KB, Shehata L, Pepper M (2020) In vivo CD4(+) T cell differentiation and function: revisiting the Th1/Th2 paradigm. Annu Rev Immunol 38:705–725
Nguyen QP, Deng TZ, Witherden DA, Goldrath AW (2019) Origins of CD4(+) circulating and tissue-resident memory T-cells. Immunology 157(1):3–12
Chatzileontiadou DS, Sloane H, Nguyen AT, Gras S, Grant EJ (2020) The many faces of CD4(+) T cells: immunological and structural characteristics. Int J Mol Sci 22(1):73
Rahimi K, Ahmadi A, Hassanzadeh K et al (2019) Targeting the balance of T helper cell responses by curcumin in inflammatory and autoimmune states. Autoimmun Rev 18(7):738–748
Støy S, Laursen TL, Glavind E et al (2020) Low interleukin-22 binding protein is associated with high mortality in alcoholic hepatitis and modulates interleukin-22 receptor expression. Clin Transl Gastroenterol 11(8):e00197
Wolk K, Sabat R (2006) Interleukin-22: a novel T- and NK-cell derived cytokine that regulates the biology of tissue cells. Cytokine Growth Factor Rev 17(5):367–380
Ryba-Stanisławowska M, Werner P, Brandt A, Myśliwiec M, Myśliwska J (2016) Th9 and Th22 immune response in young patients with type 1 diabetes. Immunol Res 64(3):730–5
Xie MH, Aggarwal S, Ho W-H et al (2000) Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2-4 and IL-22R. J Biol Chem 275(40):31335–9
Jia L, Wu C (2014) The biology and functions of Th22 cells. Adv Exp Med Biol 841:209–230
Xuan X, Zhang L, Tian C et al (2021) Interleukin-22 and connective tissue diseases: emerging role in pathogenesis and therapy. Cell Biosci 11(1):2
Wu PW, Li J, Kodangattil SR et al (2008) IL-22R, IL-10R2, and IL-22BP binding sites are topologically juxtaposed on adjacent and overlapping surfaces of IL-22. J Mol Biol 382(5):1168–83
de Moura PR, Watanabe L, Bleicher L et al (2009) Crystal structure of a soluble decoy receptor IL-22BP bound to interleukin-22. FEBS Lett 583(7):1072–7
Lücke J, Sabihi M, Zhang T et al (2021) The good and the bad about separation anxiety: roles of IL-22 and IL-22BP in liver pathologies. Semin Immunopathol 43(4):591–607
Logsdon NJ, Jones BC, Josephson K, Cook J, Walter MR (2002) Comparison of interleukin-22 and interleukin-10 soluble receptor complexes. J Interferon Cytokine Res 22(11):1099–112
Wolk K, Witte E, Hoffmann U et al (2007) IL-22 induces lipopolysaccharide-binding protein in hepatocytes: a potential systemic role of IL-22 in Crohn’s disease. J Immunol 178(9):5973–81
Mühl H, Bachmann M (2019) IL-18/IL-18BP and IL-22/IL-22BP: two interrelated couples with therapeutic potential. Cell Signal 63:109388
Dumoutier L, Lejeune D, Colau D, Renauld J-C (2001) Cloning and characterization of IL-22 binding protein, a natural antagonist of IL-10-related T cell-derived inducible factor/IL-22. J Immunol 166(12):7090–5
Zenewicz LA (2021) IL-22 Binding protein (IL-22BP) in the regulation of IL-22 biology. Front Immunol 12:766586
Chang H, Hanawa H, Liu H et al (2006) Hydrodynamic-based delivery of an interleukin-22-Ig fusion gene ameliorates experimental autoimmune myocarditis in rats. J Immunol 177(6):3635–43
Huber S, Gagliani N, Zenewicz LA et al (2012) IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature 491(7423):259–63
Lindahl H, Guerreiro-Cacais AO, Bedri SK et al (2019) IL-22 binding protein promotes the disease process in multiple sclerosis. J Immunol 203(4):888–898
Pan S, Yang D, Zhang J et al (2018) Temporal expression of interleukin-22, interleukin-22 receptor 1 and interleukin-22-binding protein during experimental periodontitis in rats. J Periodontal Res 53(2):250–257
Dumoutier L, Louahed J, Renauld JC (2000) Cloning and characterization of IL-10-related T cell-derived inducible factor (IL-TIF), a novel cytokine structurally related to IL-10 and inducible by IL-9. J Immunol 164(4):1814–1819
Prinz M, Priller J (2017) The role of peripheral immune cells in the CNS in steady state and disease. Nat Neurosci 20(2):136–144
Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K (2004) Sabat R IL-22 increases the innate immunity of tissues. Immunity 21(2):241–54
Sabat R, Ouyang W, Wolk K (2014) Therapeutic opportunities of the IL-22-IL-22R1 system. Nat Rev Drug Discov 13(1):21–38
Ouyang W, Rutz S, Crellin NK, Valdez PA (2011) Hymowitz SG regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol 29:71–109
Populin L, Stebbing MJ, Furness JB (2021) Neuronal regulation of the gut immune system and neuromodulation for treating inflammatory bowel disease. FASEB Bioadv 3(11):953–966
Witte E, Witte K, Warszawska K, Sabat R (2010) Wolk K Interleukin-22: a cytokine produced by T, NK and NKT cell subsets, with importance in the innate immune defense and tissue protection. Cytokine Growth Factor Rev 21(5):365–79
Kebir H, Kreymborg K, Ifergan I et al (2007) Human TH17 lymphocytes promote blood–brain barrier disruption and central nervous system inflammation. Nat Med 13(10):1173–5
Chen H, Tang X, Li J et al (2022) IL-17 crosses the blood–brain barrier to trigger neuroinflammation: a novel mechanism in nitroglycerin-induced chronic migraine. J Headache Pain 23(1):1
Myoung J, Kang HS, Hou W, Meng L, Dal Canto MC, Kim BS (2012) Epitope-specific CD8+ T cells play a differential pathogenic role in the development of a viral disease model for multiple sclerosis. J Virol 86(24):13717–28
Perriard G, Mathias A, Enz L et al (2015) Interleukin-22 is increased in multiple sclerosis patients and targets astrocytes. J Neuroinflammation 12:119
Sonnenberg GF, Fouser LA, Artis D (2011) Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol 12(5):383–390
Martorana A, Di Lorenzo F, Belli L et al (2015) Cerebrospinal fluid Aβ42 levels: when physiological become pathological state. CNS Neurosci Ther 21(12):921–5
Motta C, Finardi A, Toniolo S et al (2020) Protective role of cerebrospinal fluid inflammatory cytokines in patients with amnestic mild cognitive impairment and early Alzheimer’s disease carrying apolipoprotein e4 genotype. J Alzheimers Dis 76(2):681–689
Shi Y, Yamada K, Liddelow SA et al (2017) ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature 549(7673):523–527
Michelucci A, Heurtaux T, Grandbarbe L, Morga E (2009) Heuschling characterization of the microglial phenotype under specific pro-inflammatory and anti-inflammatory conditions: effects of oligomeric and fibrillar amyloid-beta. J Neuroimmunol 210(1–2):3–12
Acknowledgements
This project was supported by grants from China Postdoctoral Science Foundation (No. 2017M612870) and Natural Science Foundation of Liaoning Province (No. 2019-ZD-0807).
Author information
Authors and Affiliations
Contributions
XB-W and SX-Y contributed to experiments, data collection, manuscript writing. WQ-L, LP, LP-Z, YF-W, CF, and LM provide help for experimental testing and theoretical support. QY, XH-W, and YH help collect experimental data. XZ-L and ZF-Z contributed to experimental conception, data interpretation and manuscript revision. All authors have read and approved the submission and publication of the final version of manuscript. The authors vouch for the accuracy and completeness of the experiment.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethics approval
Reports of animal experiments state that the “Principles of laboratory animal care” (NIH publication No. 86-23, revised 1985) were followed, and was reviewed and approved by the Laboratory Animal Ethics Committee of Jinzhou Medical University.
Additional information
Managed by Massimo Federici.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, X., Yu, S., Liu, W. et al. Relationship between IL-22 and IL-22BP in diabetic cognitive dysfunction. Acta Diabetol 60, 631–644 (2023). https://doi.org/10.1007/s00592-022-02024-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-022-02024-5