Abstract
Aims
To expand the evidence base for the clinical use of metformin, we conducted a meta-analysis of randomized controlled trials (RCTs) comparing the efficacy and safety of metformin versus insulin with respect to short-term neonatal outcomes.
Methods
A comprehensive search of electronic databases (PubMed, Embase, Cochrane Library, and Web of Science) was performed. Two reviewers extracted the data and calculated pooled estimates by use of a random-effects model. In total, 24 studies involving 4355 participants met the eligibility criteria and were included in the quantitative analyses.
Results
Unlike insulin, metformin lowered neonatal birth weights (mean difference − 122.76 g; 95% confidence interval [CI] − 178.31, − 67.21; p < 0.0001), the risk of macrosomia (risk ratio [RR] 0.68; 95% CI 0.54, 0.86; p = 0.001), the incidence of neonatal intensive care unit admission (RR 0.73; 95% CI 0.61, 0.88; p = 0.0009), and the incidence of neonatal hypoglycemia (RR 0.65; 95% CI 0.52, 0.81; p = 0.0001). Subgroup analysis based on the maximum daily oral dose of metformin indicated that metformin-induced neonatal birth weight loss was independent of the oral dose.
Conclusions
Our meta-analysis provides further evidence that metformin is a safe oral antihyperglycemic drug and has some benefits over insulin when used for the treatment of gestational diabetes, without an increased risk of short-term neonatal adverse outcomes. Metformin may be particularly useful in women with gestational diabetes at high risk for neonatal hypoglycemia, women who want to limit maternal and fetal weight gain, and women with an inability to afford or use insulin safely.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gestational diabetes mellitus (GDM) is a common complication during pregnancy and is defined as any glucose intolerance that occurs or is diagnosed for the first time during pregnancy [1]. GDM develops in about 5% to 14% of all pregnancies and is associated with certain pregnancy-related complications and a long-term risk of diabetes in both the mother and offspring [2]. With the establishment of the two-child policy and epidemic of obesity in China, the incidence of GDM has been increasing, resulting in a heavy economic burden on the public health care system and individuals [3]. According to the latest data reported by the International Diabetes Federation in 2021, about one in six live births (20 million) is affected by high plasma glucose concentration during pregnancy, and GDM accounts for 83.6% of these cases of hyperglycemia [4].
Women with uncontrolled GDM have higher-risk pregnancies, and some adverse effects of GDM may also affect the fetus, including fetal anomalies, macrosomia (birth weight of > 4000 g), fetal distress, metabolic disorders, growth imbalance, hyperbilirubinemia, and some long-term complications [5]. Traditionally, insulin has been the gold standard for the treatment of GDM because it cannot cross the placenta and allows for precise glucose control. However, insulin therapy has several disadvantages, including the need for multiple injections, risks of hypoglycemia and hyperbilirubinemia, the rising cost of insulin, and the lack of affordability [6]. These disadvantages suggest that current treatment regimens fall short of optimizing outcomes. Metformin is a commonly used oral antihyperglycemic drug in clinical practice with excellent efficacy in terms of glycemic control and weight loss, good tolerance, and a reasonable price [7]. Several organizations currently support its use as an alternative to insulin [8, 9]. However, recent long-term studies of offspring have provided conflicting results. Two follow-up studies of children aged 2 to 9 years whose mothers had gestational diabetes showed that several growth parameters tended to be larger in metformin-exposed offspring than in offspring exposed to insulin. These growth parameters included weight, body mass index, triceps skinfold, waist and arm circumferences and body fat percent, and they were also associated with cardio-metabolic disease in later life [10, 11]. This has slowed the clinical use of metformin as a substitute for insulin in the treatment of GDM.
We therefore performed this updated meta-analysis to compare the efficacy and safety of metformin versus insulin with respect to short-term neonatal outcomes in the treatment of GDM. The objective of our study was to determine whether metformin is superior to insulin in terms of altering neonatal growth outcomes and inducing neonatal adverse outcomes during treatment of GDM. Addressing this issue is particularly important because the number of pregnancies exposed to metformin is increasing worldwide.
Methods
This systematic review and meta-analysis are reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement and was registered at the International Prospective Register of Systematic Reviews (CRD42022330187) [12].
Search strategy
A systematic literature search of PubMed, Embase, the Cochrane Library, and Web of Science (last search was updated on 1 May 2022) was performed using prespecified terms (Supplemental Text S1) with no filters and no language or location restrictions. We also searched for additional eligible trials in previously published meta-analyses on related topics.
Inclusion and exclusion criteria
Studies that met the following criteria were included: (1) The population comprised pregnant women with GDM, (2) The interventions were metformin (with or without extra insulin treatment) and insulin, (3) The study included one or more neonatal outcomes, and (4) The study design was a randomized controlled trial (RCT). We excluded studies involving pregnant women with pre-existing diabetes, and duplicate studies published in different journals were included only once.
Definitions of neonatal outcomes
The neonatal outcomes included neonatal growth outcomes and neonatal adverse outcomes. The neonatal growth outcomes were birth weight, birth height, macrosomia (≥ 4000 g), large for gestational age (LGA) (birth weight at the > 90th percentile), and small for gestational age (SGA) (birth weight at the < 10th percentile). The neonatal adverse outcomes were neonatal hypoglycemia, admission to the neonatal intensive care unit (NICU), hyperbilirubinemia, respiratory distress syndrome, premature birth, congenital anomalies, abnormal pH of the umbilical cord, abnormal Apgar score at 5 min, neonatal death, neonatal sepsis, and birth trauma.
Data collection and management
The titles, abstracts, citation information, and descriptor terms of the publications identified through the search strategy were screened. Full-text articles of all selected abstracts were obtained, and two reviewers (Bo Sheng and Juan Ni) independently assessed all the full-text articles for eligibility to determine the final study selection. Any disagreements between the two authors were settled by group discussion until a consensus was reached. We designed a data extraction form to collect relevant information including the authors, year of publication, country, number of patients, definition of gestational diabetes, patient characteristics, and interventions.
Risk of bias and quality assessment
We used the Cochrane Collaboration’s tool to assess the risk of bias in terms of the following seven aspects: (1) Random sequence generation (selection bias), (2) Allocation concealment (selection bias), (3) Blinding of participants and personnel (performance bias), (4) Blinding of outcome assessment (detection bias), (5) Incomplete outcome data (attrition bias), (6) Selective reporting (reporting bias), and (7) Other bias. We classified these aspects as low risk of bias, uncertain risk of bias, or high risk of bias.
We assessed the quality of evidence in these studies by using the GRADE profiler (GRADEpro GDT) [13]. The GRADE system was used to assess the study limitations (risk of bias), inconsistency, indirectness, imprecision, and publication bias across the body of evidence to derive an overall summary of the quality of evidence, which was classified each as high, moderate, low, or very low.
Statistical analysis
The standardized mean difference (SMD) was calculated using the mean and standard deviation for continuous variables. The risk ratio (RR) was calculated for dichotomous variables with 95% confidence intervals (CIs). The meta-analysis was performed using Review Manager (RevMan) version 5.4.1 (Nordic Cochrane Centre, Copenhagen, 2014), and Egger’s test was used to assess publication bias through the ‘metafor’ package in R version 3.5.1 [14]. The studies were determined to be heterogenous if I2 > 50% and p < 0.1. A sensitivity analysis was performed by excluding each study one by one to evaluate the credibility of the pooled results. A prespecified subgroup analysis was also performed to explore the sources of heterogeneity. Potential publication bias was assessed by the application of contour-enhanced funnel plots and Egger’s linear regression test at the p < 0.05 level of significance. If publication bias was indicated, we further evaluated the number of missing studies by trim-and-fill analysis and recalculated the pooled risk estimate with the addition of those missing studies. Except where otherwise specified, a p value of < 0.05 was considered statistically significant.
Results
Literature search and study characteristics
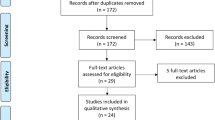
In total, 576 studies were retrieved through PubMed, Embase, the Cochrane Library, and Web of Science. After removal of duplicates and title/abstract screening, 188 trials underwent full text assessment, after which the full set of eligibility criteria was applied. After full text evaluation, 24 studies remained eligible for inclusion in this review. The process of study selection is illustrated in Fig. 1. As shown in Table 1, 24 RCTs involving 4355 patients with GDM were included to estimate the impact of metformin versus insulin on neonatal outcomes [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38]. The earliest study began in 2001, and the latest study was completed in 2021. Five studies each were conducted in Iran [23, 30,31,32, 35],Egypt [15, 18, 20, 22, 37], and Pakistan [16, 17, 21, 27, 38]; three in Finland [26, 28, 34]; and one each in Australia [19], India [36], Spain [29], Brazil [33], New Zealand [24], and the USA [25]. In this meta-analysis, we mainly focused on the daily oral dose of metformin in pregnant women with GDM. Three studies among the 24 RCTs did not report the dose of metformin [19, 23, 30], and the remaining 21 studies were included for further subgroup analysis.
Supplemental Fig. S1 provides a summary of the risk of bias for each included study. No selection bias, attrition bias, or selective bias was present in any of the RCTs, indicating relatively high quality. Because insulin was given by injection and metformin was given orally, all the included studies involved open allocation, which did not affect the short-term neonatal outcomes because these were all objective. The quality of the evidence (GRADE) for the neonatal outcomes of interest, including neonatal birth weight, macrosomia, LGA, SGA, birth height, NICU admission, and neonatal hypoglycemia, was very low to moderate. The GRADE system evidence for the above outcomes and reasons for upgrade and downgrade are shown in Table 2.
Neonatal birth weight and macrosomia
Twenty-two studies involving 4174 neonates reported the neonatal birth weight. [15,16,17,18,19, 21,22,23, 25,26,27,28,29,30,31,32,33,34,35,36,37,38] The results indicated that the birth weights of neonates whose mothers were treated with metformin were significantly lower than those of neonates whose mothers were treated with insulin during pregnancy (95% CI − 178.31, − 67.21; I2 = 84%; p < 0.0001) (Fig. 2A). On average, metformin-exposed neonates weighed 122.76 g less than those whose mothers received insulin. Similar to the birth weight in the metformin-exposed group, metformin also lowered the risk of macrosomia by 30% compared with the insulin-exposed group based on 20 studies (RR 0.75; 95% CI 0.54, 0.86; I2 = 17%; p = 0.001) (Fig. 2B) [15, 17, 18, 20, 22, 24,25,26,27,28,29,30,31,32,33,34,35,36,37,38].
To explore potential source of heterogeneity among the studies, we carried out several sensitivity analyses (Supplemental Fig. S2). Nevertheless, significant heterogeneity (I2 = 74%) was still present among the studies after we excluded one study from the analysis [21]. Next, 20 studies involving 3408 neonates were included in a subgroup analysis of birth weight [15,16,17,18, 21, 22, 24,25,26,27,28,29,30,31,32,33,34,35, 37, 38], and we found that the neonates whose mothers were treated with a maximum oral dosage of metformin of 1500 mg/day (95% CI − 363.51, − 80.06; I2 = 0%; p = 0.002), 2500 mg/day (95% CI − 198.89, − 76.63; I2 = 68%; p < 0.001), and 3000 mg/day (95% CI − 337.96, − 62.04; p = 0.004) had obviously lower birth weights than those of neonates whose mothers were treated with insulin. However, the birth weight of neonates born to mothers treated with a maximum oral dosage of metformin of 2000 mg/day (95% CI − 342.44, 139.67; I2 = 92%; p = 0.41) and 2250 mg/day (95% CI − 150.62, 69.37; I2 = 0%; p = 0.47) showed no significant difference between the groups (Fig. 3).
To assess the potential publication bias of neonatal birth weight, we used the ‘metafor’ package of R software for Egger’s test. Our results showed that the funnel plot of neonatal birth weight was asymmetrical (Supplemental Fig. S3A), and Egger’s test indicated possible publication bias (p = 0.008) (Supplemental Table S1). Next, we used trim-and-fill analysis to recalculate our pooled risk estimate; the results suggested no publication bias (p = 0.28), and the funnel plot also became symmetrical (Supplemental Fig. S3B).
Other neonatal growth outcomes
Twelve studies reported the frequency of LGA and SGA [17, 19, 20, 22, 24, 26, 28, 29, 31,32,33,34], and three studies reported the neonatal height [24, 29, 31]. The results suggested no difference in the risk of being born LGA (RR 0.86; 95% CI 0.73, 1.02; I2 = 0%; p = 0.08), the risk of being born SGA (RR 1.00; 95% CI 0.77, 1.30; I2 = 0%; p = 1.0), or the neonatal height (95% CI − 0.67, 0.19; I2 = 38%; p = 0.27) between metformin and insulin exposure (Fig. 4). No evidence of publication bias was observed by Egger’s test in other neonatal growth outcomes (Supplemental Fig. S3 and Table S1).
Neonatal adverse outcomes
Eighteen studies involving 3527 neonates reported the incidence of NICU admission [15, 17, 19, 20, 22,23,24,25,26,27,28,29, 31, 32, 34,35,36, 38], and the results indicated a lower incidence in metformin-exposed than insulin-exposed neonates (RR 0.73; 95% CI, 0.61, 0.88; I2 = 23%; p = 0.0009) (Fig. 5A). Moreover, 20 studies involving 3670 neonates were included in the analysis of neonatal hypoglycemia [15, 17, 18, 20, 22,23,24,25,26,27,28,29,30,31,32,33,34,35,36, 38]. The results showed that insulin-exposed neonates had a higher incidence of hypoglycemia than metformin-exposed neonates (RR 0.65; 95% CI 0.52, 0.81; I2 = 22%; p = 0.0001) and that metformin lowered the risk of neonatal hypoglycemia by 45% compared with the insulin-exposed group (Fig. 5B). We used contour-enhanced funnel plots and Egger’s linear regression test to assess the potential publication bias of NICU admission and neonatal hypoglycemia (Supplemental Fig. S3D and S3F). Egger’s test indicated no publication bias for NICU admission, but neonatal hypoglycemia was associated with possible publication bias (p = 0.006) (Supplemental Table S1). We used trim-and-fill analysis to recalculate our pooled risk estimate of neonatal hypoglycemia, which suggested no publication bias (p = 0.71) (Supplemental Fig. S3E).
There were no significant differences in the other neonatal adverse outcomes, including respiratory distress syndrome (14 studies) (RR 0.71; 95% CI, 0.51, 0.99; I2 = 0%; p = 0.07), an abnormal Apgar score at 5 min (15 studies) (RR 0.00; 95% CI − 0.15, 0.16; I2 = 59%; p = 0.95), hyperbilirubinemia (9 studies) (RR 0.88; 95% CI 0.69, 1.12; I2 = 0%; p = 0.29), congenital anomalies (9 studies) (RR 0.73; 95% CI 0.44, 1.22; I2 = 0%; p = 0.23), preterm birth (11 studies) (RR 1.08; 95% CI 0.78, 1.50; I2 = 22%; p = 0.63), an abnormal pH of the umbilical cord (5 studies) (RR 0.01; 95% CI − 0.00, 0.01; I2 = 0%; p = 0.14), neonatal death (10 studies) (RR 0.52; 95% CI 0.13, 2.18; I2 = 0%; p = 0.37), neonatal sepsis (4 studies) (RR 0.71; 95% CI 0.34, 1.45; I2 = 0%; p = 0.34), and birth trauma (6 studies) (RR 0.92; 95% CI 0.57, 1.49; I2 = 0%; p = 0.74) (Supplemental Figs. S4 and S5).
Discussion
In this systematic review and meta-analysis, we found that neonates exposed to metformin in utero weighed less at birth than those whose mothers were exposed to insulin. The risk of macrosomia is substantially lower (by 30%) when GDM is treated with metformin than with insulin, and there is no concomitant increase in the risk of being born SGA or LGA. Despite being born at lower average birth weights, neonates of metformin-treated women do not have an increased incidence of neonatal adverse outcomes. In contrast, metformin significantly lowers the risk of neonatal hypoglycemia and the incidence of NICU admission.
It is well accepted that the fetuses of obese women with GDM have a higher risk of developing macrosomia than those of women with GDM of normal weight [39]. Some recent meta-analyses showed that weight gain during pregnancy was significantly lower in women with GDM who received metformin than in those who received insulin [4, 7]. Whether metformin-induced weight loss in women with GDM leads to a significant reduction in the incidence of fetal macrosomia remains unclear. Our results provide evidence that metformin can also effectively control neonatal birth weight and reduce the incidence of fetal macrosomia. In particular, there is growing evidence that macrosomia is likely to be associated with shoulder dystocia, brachial plexus injury, delayed motor development, and a higher risk of obesity or diabetes later in life [7, 40]. Moderate neonatal birth weight control may effectively reduce and avoid some complications related to macrosomia, especially for pregnant women with GDM. To explore the relationship between neonatal birth weight and the oral dose of metformin, we performed a subgroup analysis of neonatal birth weight based on the maximum daily oral dose of metformin. We found that a maximum oral dosage of metformin of 1500, 2500, and 3000 mg/day was associated with neonatal birth weight loss, but there was no significant difference in an oral dosage of metformin of 2000 and 2250 mg/day. These results suggest that metformin-induced neonatal birth weight loss occurs independently of the oral dose of metformin. This is consistent with the previous finding that a low dosage of metformin (< 1000 mg/day), but not a high dosage, had significant efficacy for body mass index control or weight loss in adolescents [41].
Macrosomic fetuses in women with diabetes develop a unique pattern of overgrowth involving central deposition of subcutaneous fat in the abdominal and interscapular areas with skeletal growth remaining largely unaffected [40, 42]. During early gestation, the embryo expresses very low levels of organic cation transporters, making metformin likely to be safe in the first trimester. However, metformin can easily cross the placenta via organic cation transporters in the second and third trimesters and may reach near-maternal concentrations in the fetus [43]. In addition to lowering blood glucose concentration, metformin has a variety of intracellular effects including inhibition of mitochondrial respiration and effects on the nutrient-sensing pathway by both adenosine monophosphate-activated protein kinase and mammalian target of rapamycin mechanisms [44,45,46,47]. Moreover, in the Metformin in Women with Type 2 Diabetes in Pregnancy (MiTy) trial, the lower neonatal adiposity in the metformin group led to a lower incidence of fetal macrosomia [48]. Therefore, the significant metformin-induced reduction in the incidence of macrosomia may be related to the inhibition of fetal fatty acid synthesis. This effect of metformin differentiates its dose-dependent hypoglycemic effect, the underlying mechanism of which remains to be explored.
In accordance with previous meta-analyses [7, 51, 52], the incidence of NICU admission and hypoglycemia were also significantly reduced in our study. The rates of NICU admission are mainly influenced by fetal physiologic compromise, including preterm birth, hypoglycemia, respiratory distress syndrome, and neonatal jaundice. In our meta-analysis, the infants born to mothers treated with insulin needed additional management for hypoglycemia, which is partly associated with an increase in NICU admission. Neonatal hypoglycemia is one of the most common metabolic disorders of the newborn and is due to hyperinsulinemia of the fetus in response to maternal hyperglycemia in utero [49]. Fetal hypoglycemia can also lead to more serious complications such as seizures and serious brain injury [50]. Notably, metformin significantly lowered the risk of neonatal hypoglycemia by 44% in our meta-analysis, and it may reduce the risk of neonatal brain injury. The use of metformin may not harm the fetus during pregnancy and may be safer in the neonatal period with potentially beneficial effects.
A major strength of our meta-analysis is our provision of a complete overview of the effect of maternal metformin exposure on neonatal growth outcomes and neonatal adverse outcomes. We included 24 studies, which is a higher number than included in previous analyses; additionally, all of these studies were RCTs, which greatly reduced the likelihood of recall and selection biases. Moreover, a subgroup analysis by the different daily doses of metformin for treatment of GDM and an investigation of the relationship between the maternal oral dose of metformin and neonatal birth weight were carried out for the first time. Furthermore, we assessed potential publication bias by contour-enhanced funnel plots and Egger’s test, the results of which suggested that our results regarding neonatal outcomes were not affected by publication bias. This increases the confidence in our findings.
Our study has several limitations that merit further discussion. First, the possibility of confounding factors in several studies cannot be completely ruled out. For example, women who had poor glycemic control with metformin and required extra insulin therapy were included in the metformin-treated group in some studies, which might cause selection bias. However, the proportion of metformin-treated women requiring insulin supplementation ranged from 8.6% to 46.8% (average, 16.2%) of the total metformin-treated women. Moreover, these patients used a lower total insulin dose than those treated with insulin alone. Therefore, we believe that such selection bias may not have influenced the overall outcomes of the studies. Second, data on neonatal growth outcomes and neonatal adverse outcomes were unavailable or incompletely reported in most of the included studies, restricting us from performing a more detailed relevant analysis and obtaining more comprehensive results. Finally, although subgroup and sensitivity analyses were performed to explore the potential sources of heterogeneity in neonatal birth weight, the cause of the high heterogeneity remains unclear.
In conclusion, the results of this meta-analysis add to the evidence that metformin may be particularly useful in women with GDM at high risk for neonatal hypoglycemia, women who want to limit maternal and fetal weight gain, or women with an inability to afford or use insulin safely. Metformin can effectively lower neonatal birth weight and the incidences of macrosomia, neonatal hypoglycemia, and NICU admission compared with insulin without an increased risk of neonatal adverse outcomes. Whether the effect of metformin on neonatal birth weight is associated with the oral dose of metformin requires further investigation in large-scale trials.
Availability of data and materials
Data will be available upon request of the corresponding author.
References
Alwan N, Tuffnell DJ, West J (2009) Treatments for gestational diabetes. Cochrane Database Syst Rev 3:1–57
Canningham FG, Leveno KJ, Bloom SL et al (2019) Williams obstetrics, 25th edn. McGraw-Hill, New York, pp 1104–1121
Guariguata L, Whiting D, Weil C, Unwin N (2011) The International Diabetes Federation diabetes atlas methodology for estimating global and national prevalence of diabetes in adults. Diabetes Res Clin Pract 94(3):322–332
Balsells M, García-Patterson A, Solà I et al (2015) Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ 350:h102
Butalia S, Gutierrez L, Lodha A et al (2017) Short- and long-term outcomes of metformin compared with insulin alone in pregnancy: a systematic review and meta-analysis. Diabet Med 34(1):27–36
Poolsup N, Suksomboon N, Amin M (2014) Effect of treatment of gestational diabetes mellitus: a systematic review and meta-analysis. PLoS ONE 9(3):e92485
Bao LX, Shi WT, Han YX (2021) Metformin versus insulin for gestational diabetes: a systematic review and meta-analysis. J Matern Fetal Neonatal Med 34(16):2741–2753
Society of Maternal-Fetal Medicine Publications Committee (2018) SMFM statement: pharmacological treatment of gestational diabetes. Am J Obstet Gynecol 218(5): B2–B4
Feig DS, Berger H, Donovan L et al (2018) Diabetes canada clinical practice guidelines expert committee. Diabetes Pregnancy Can J Diabetes 42:S255–S282
Rowan JA, Rush EC, Obolonkin V et al (2011) Metformin in gestational diabetes: the offspring follow-up (MiG TOFU)—body composition at 2 years of age. Diabetes Care 34(10):2279–2284
Rowan JA, Rush EC, Plank LD et al (2018) Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition and metabolic outcomes at 7–9 years of age. BMJ Open 6(1):e000456
Moher D, Liberati A, Tetzlaff J et al (2009) PRISMA Group, Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 6(7):e1000097
Balshem H, Helfand M, Schünemann HJ et al (2011) GRADE guidelines: 3 rating the quality of evidence. J Clin Epidemiol 64(4):401–406
R Core Team (2018) R: a language and environment for statistical computing. Version 3.5.1. Vienna, R Foundation for Statistical Computing.
Ahmed AKH, Galal SKH, Abdelaziz EA (2021) Metformin versus insulin in gestational diabetes. Med J Cairo Univ 89(6):2525–2532
Arshad R, Khanam S, Shaikh F et al (2017) Feto-maternal outcomes and glycemic control in metformin versus insulin treated gestational diabetics. Pak J Med Sci 33(5):1182–1187
Ainuddin J, Karim N, Hasan AA et al (2015) Metformin versus insulin treatment in gestational diabetes in pregnancy in a developing country: a randomized control trial. Diabetes Res Clin Pract 107(2):290–299
Ashoush S, El-Said M, Fathi H et al (2016) Identification of metformin poor responders, requiring supplemental insulin, during randomization of metformin versus insulin for the control of gestational diabetes mellitus. J Obstet Gynaecol Res 42(6):640–647
Barrett HL, Gatford KL, Houda CM et al (2013) Maternal and neonatal circulating markers of metabolic and cardiovascular risk in the metformin in gestational diabetes (MiG) trial: responses to maternal metformin versus insulin treatment. Diabetes Care 36(3):529–536
Saleh HS, Abdelsalam WA, Mowafy HE et al (2016) Could metformin manage gestational diabetes mellitus instead of insulin? Int J Reprod Med 2016:3480629
Hamadani A, Zahid S, Butt ZB (2017) Metformin versus insulin treatment in gestational diabetes in pregnancy and their effects on neonatal birthweight. Pak J Med Sci 11:914–916
Eid SR, Moustafa RSI, Salah MM et al (2018) Is metformin a viable alternative to insulin in the treatment of gesta- tional diabetes mellitus (GDM)? Comparison of maternal and neonatal outcomes. Egypt Pediatr Assoc Gaz 66(1):15–21
Ghomian N, Vahed SHM, Firouz S et al (2019) The efficacy of metformin compared with insulin in regulating blood glucose levels during gestational diabetes mellitus: a randomized clinical trial. J Cell Physiol 234(4):4695–4701
Rowan JA, Hague WM, Gao W et al (2008) Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med 358(19):2003–2015
Moore LE, Briery CM, Clokey D et al (2007) Metformin and insulin in the management of gestational diabetes mellitus: preliminary results of a comparison. J RAeprod Med 52(11):1011–1015
Ijäs H, Vääräsmäki M, Morin-Papunen L et al (2011) Metformin should be considered in the treatment of gestational diabetes: a prospective randomised study. BJOG 118(7):880–885
Hassan JA, Karim N, Sheikh Z (2012) Metformin prevents macrosomia and neonatal morbidity in gestational diabetes. Pak J Med Sci 28(3):384–389
Huhtala MS, Tertti K, Juhila J et al (2020) Metformin and insulin treatment of gestational diabetes: effects on inflammatory markers and IGF-binding protein-1-secondary analysis of a randomized controlled trial. BMC Pregnancy Childbirth 20(1):401
Picón-César MJ, Molina-Vega M, Suárez-Arana M et al (2021) Metformin for gestational diabetes study: metformin vs insulin in gestational diabetes: glycemic control and obstetrical and perinatal outcomes: randomized prospective trial. Am J Obstet Gynecol 225(5):e1–e517
Jahanshahi M, Shahmirzadi AR, Kashani E et al (2020) Effects of metformin and insulin therapy regimens on postpartum oral glucose tolerance test results in pregnant women with gestational diabetes mellitus: a comparative study. Horm Mol Biol Clin Investig 41(4):2020018
Niromanesh S, Alavi A, Sharbaf FR et al (2012) Metformin compared with insulin in the management of gestational diabetes mellitus: a randomized clinical trial. Diabetes Res Clin Pract 98(3):422–429
Mesdaghinia E, Samimi M, Homaei Z et al (2013) Comparison of newborn outcomes in women with gestational diabetes mellitus treated with metformin or insulin: a randomised blinded trial. Int J Prev Med 4(3):327–333
Spaulonci CP, Bernardes LS, Trindade TC et al (2013) Randomized trial of metformin vs insulin in the management of gestational diabetes. Am J Obstet Gynecol 209(1):e1–e7
Tertti K, Ekblad U, Koskinen P et al (2013) Metformin vs. insulin in gestational diabetes: A randomized study characterizing metformin patients needing additional insulin. Diabetes Obes Metab 15(3):246–251
Ruholamin S, Eshaghian S, Allame Z (2014) Neonatal outcomes in women with gestational diabetes mellitus treated with metformin in compare with insulin: a randomized clinical trial. J Res Med Sci 19(10):970–975
Somani PS, Sahana PK, Chaudhuri P et al (2016) Treatment of gestational diabetes mellitus: insulin or metformin? J Evolution Med Dent 5(63):4423–4429
Gamal HE, Elaleem MA, Sadek S et al (2018) Insulin versus metformin in treatment of gestational diabetes mellitus (randomized controlled clinical trial). Egypt J Hosp Med 72(1):3753–3761
Wasim T, Shaukat S, Javaid L et al (2019) Comparison of metformin and insulin for management of gestational diabetes mellitus: a randomized control trial. Pak J Med Health Sci 13(3):823–827
Yogev Y, Langer O (2008) Pregnancy outcome in obese and morbidly obese gestational diabetic women. Eur J Obstet Gynecol Reprod Biol 137(1):21–26
Kc K, Shakya S, Zhang H (2015) Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab 66(2):14–20
Hui F, Zhang Y, Ren T et al (2019) Role of metformin in overweight and obese people without diabetes: a systematic review and network meta-analysis. Eur J Clin Pharmacol 75(4):437–450
Tarry-Adkins JL, Aiken CE, Ozanne SE (2019) Neonatal, infant, and childhood growth following metformin versus insulin treatment for gestational diabetes: a systematic review and meta-analysis. PLoS Med 16(8):e1002848
Lee N, Hebert MF, Prasad B et al (2013) Effect of gestational age on mRNA and protein expression of polyspecific organic cation transporters during pregnancy. Drug Metab Dispos 41(12):2225–2232
Owen MR, Doran E, Halestrap AP (2000) Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J 348(3):607–614
Howell JJ, Hellberg K, Turner M et al (2017) Metformin inhibits hepatic mTORC1 signaling via dose-dependent mechanisms involving AMPK and the TSC complex. Cell Metab 25(2):463–471
Ben Sahra I, Regazzetti C, Robert G et al (2011) Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res 71(13):4366–4372
Jansson N, Rosario FJ, Gaccioli F et al (2013) Activation of placental mTOR signaling and amino acid transporters in obese women giving birth to large babies. J Clin Endocrinol Metab 98(1):105–113
Feig DS, Murphy K, Asztalos E et al (2016) MiTy Collaborative Group. Metformin in women with type 2 diabetes in pregnancy (MiTy): a multicenter randomized controlled trial. BMC Pregnancy Childbirth 16(1):173
Kole MB, Ayala NK, Clark MA et al (2020) Factors associated with hypoglycemia among neonates born to mothers with gestational diabetes mellitus. Diabetes Care 43(12):e194–e195
Harding JE, Harris DL, Hegarty JE et al (2017) An emerging evidence base for the management of neonatal hypoglycemia. Early Hum Dev 104:51–56
Su DF, Wang XY (2014) Metformin vs insulin in the management of gestational diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract 104(3):353–357
Jiang YF, Chen XY, Ding T et al (2015) Comparative efficacy and safety of OADs in management of GDM: network meta-analysis of randomized controlled trials. J Clin Endocrinol Metab 100(5):2071–2080
Acknowledgements
We thank Angela Morben, DVM, ELS, from Liwen Bianji (Edanz) (www.liwenbianji.cn), for editing the English text of a draft of this manuscript.
Funding
No specific funding was received for this study.
Author information
Authors and Affiliations
Contributions
BS, JN, and BL: Conception of research protocol, study design, literature review, data extraction and interpretation. BS: Drafting of the manuscript. HL and XML: Manuscript revision and project development. GGJ: Data analysis and quality assessment. All authors have read and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical Standard Statement
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
All procedures in this study were conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975.
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
As the corresponding author, I confirm on behalf of all authors that there have been no involvements that might raise the question of bias in the work reported or in the conclusions, implications, or opinions stated.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the topical collection Pregnancy and Diabetes, managed by Antonio Secchi and Marina Scavini.
Supplementary Information
Below is the link to the electronic supplementary material.
592_2022_2016_MOESM1_ESM.docx
Text S1. Database search strategies. (A) PubMed. (B) Ovid Embase. (C) Web of Science. (D) Cochrane Library. (DOCX 21 kb)
592_2022_2016_MOESM3_ESM.tif
Fig. S1. Summary of risk of bias for each included study. +, low risk of bias; ?, unclear risk; -, high risk. (TIF 4812 kb)
592_2022_2016_MOESM4_ESM.pdf
Fig. S2. Leave-one-out sensitivity analysis of neonatal birth weight. Data are expressed as mean difference (random-effects model) and 95% CI. (PDF 936 kb)
592_2022_2016_MOESM5_ESM.pdf
Fig. S3. Funnel plots to assess publication bias of neonatal outcomes. (A) Neonatal birth weight. (B) Neonatal birth weight after trim-and-fill analysis. (C) Macrosomia. (D) Neonatal hypoglycemia. (E) Neonatal hypoglycemia after trim-and-fill analysis. (F) NICU admission. (G) LGA. (H) SGA. (I) Neonatal birth height. (PDF 873 kb)
592_2022_2016_MOESM6_ESM.pdf
Fig. S4. Forest plots for neonatal adverse outcomes. (A) Respiratory distress syndrome. (B) Hyperbilirubinemia. (C) Abnormal pH of umbilical cord. (F) Preterm birth. (PDF 2197 kb)
592_2022_2016_MOESM7_ESM.pdf
Fig. S5. Forest plots for neonatal adverse outcomes. (A) Apgar score at 5 minutes. (B) Congenital anomalies. (C) Neonatal death. (D) Neonatal sepsis. (E) Birth trauma. (PDF 2421 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sheng, B., Ni, J., Lv, B. et al. Short-term neonatal outcomes in women with gestational diabetes treated using metformin versus insulin: a systematic review and meta-analysis of randomized controlled trials. Acta Diabetol 60, 595–608 (2023). https://doi.org/10.1007/s00592-022-02016-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-022-02016-5