Abstract
Background
Type 2 diabetes mellitus (T2DM) has already become a global pandemic. Recently, reports showed its pathogenesis was closely related to a disorder of gut microbiota. In China, the Liu–Wei–Di–Huang Pills (LWDH) have treated T2DM for thousands of years. However, its therapeutic mechanism associated with gut microbiota is worthy of further study.
Aims
This study aims to investigate the effects of LWDH on T2DM by regulating gut microbiota and short-chain fatty acids (SCFAs) in Goto–Kakizaki (GK) rats.
Methods
T2DM models were successfully established based on GK rats and administrated with LWDH. The changes in fasting blood glucose (FBG), oral glucose tolerance test (OGTT), and serum insulin (INS) were determined, and the immunohistochemical (IHC) method was used to test INS expression in pancreas. The 16S-ribosomal DNA (16S rDNA) sequencing analysis assessed gut microbiota structural changes; a gas chromatography–mass spectrometer (GC–MS)-based metabolomics method was adopted to detect SCFA levels. The pathological morphology of jejunum was detected by hematoxylin–eosin (H&E) staining, and the expression of GPR43, GPR41, GLP-1, and GLP-1R was evaluated by qRT-PCR and ELISA, respectively.
Results
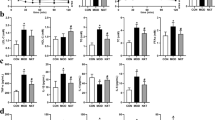
We observed that GK rats treated with LWDH: (a) has altered the microbial structure and promoted the abundance of bacteria in Firmicutes, including Lactobacillus, Allobaculum, and Ruminococcus_2, (b) increased SCFAs levels involving acetic acid, propionic acid, and butyric acid and (c) alleviated T2DM and jejunum injuries potentially based on SCFAs-GPR43/41-GLP-1 pathway.
Conclusion
LWDH could improve T2DM by regulating gut microbiota and SCFAs, and the therapeutic mechanism might be related to the SCFAs-GPR43/41-GLP-1 pathway.








Similar content being viewed by others
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
Abbreviations
- T2DM:
-
Type 2 diabetes mellitus
- LWDH:
-
Liu–Wei–Di–Huang Pills
- SCFAs:
-
Short-chain fatty acids
- GK:
-
Goto–Kakizaki
- FBG:
-
Fasting blood glucose
- OGTT:
-
Oral glucose tolerance test
- INS:
-
Serum insulin
- IHC:
-
Immunohistochemical
- 16S rDNA:
-
The16S-ribosomal DNA
- GC–MS:
-
A gas chromatography–mass spectrometer
- H&E:
-
Hematoxylin-eosin
- GLP-1:
-
Glucagon-like peptide-1
- GLP-1R:
-
Glucagon-like peptide-1 receptor
- GPCRs:
-
G-protein-coupled receptors
- GPR43:
-
G-protein-coupled receptors 43
- GPR41:
-
G-protein-coupled receptors 41
- TCM:
-
Traditional Chinese medicine
- MOD:
-
Model group
- POS:
-
Positive group
- CON:
-
Control group
- AOD:
-
Average optical density
- PCoA:
-
Principal coordinates analysis
- NMDS:
-
Non-metric multidimensional scaling
- LDA:
-
Linear discriminant analysis
- LEfSe:
-
Linear discriminant analysis effect size
- DC:
-
Degree
- BC:
-
Betweenness centrality
- CC:
-
Closeness centrality
- PICRUSt 2.0:
-
Phylogenetic Investigation of Communities by Reconstruction of Unobserved States Ver2.0
References
Zheng Y, Ley SH, Hu FB (2018) Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol 14(2):88–98
Cani PD, Amar J, Iglesias MA et al (2007) Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56(7):1761–1772
Horie M, Miura T, Hirakata S et al (2017) Comparative analysis of the intestinal flora in type 2 diabetes and nondiabetic mice. Exp Anim 66(4):405–416
Qin J, Li Y, Cai Z et al (2012) A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490(7418):55–60
Adeshirlarijaney A, Gewirtz AT (2020) Considering gut microbiota in treatment of type 2 diabetes mellitus. Gut Microbes 11(3):253–264
Gong X, Li X, Bo A et al (2020) The interactions between gut microbiota and bioactive ingredients of traditional Chinese medicines: a review. Pharmacol Res 157:104824
Feng W, Ao H, Peng C, Yan D (2019) Gut microbiota, a new frontier to understand traditional Chinese medicines. Pharmacol Res 142:176–191
Gao K, Yang R, Zhang J et al (2018) Effects of Qijian mixture on type 2 diabetes assessed by metabonomics, gut microbiota and network pharmacology. Pharmacol Res 130:93–109
Guo C, Wang Y, Zhang S et al (2021) Crataegus pinnatifida polysaccharide alleviates colitis via modulation of gut microbiota and SCFAs metabolism. Int J Biol Macromol 181:357–368
Zeng J, Cheng B, Huang Y et al (2019) Active Fraction Combination from Liuwei Dihuang Decoction (LW-AFC) alleviated the LPS-induced long-term potentiation impairment and glial cells activation in hippocampus of mice by modulating immune responses. Evid Based Complement Alternat Med 2019:3040972
Tseng YT, Chang WH, Lin CC et al (2019) Protective effects of Liuwei dihuang water extracts on diabetic muscle atrophy. Phytomedicine 53:96–106
Huang JH, He D, Chen L et al (2020) A GC–MS-based metabolomics investigation of the protective effect of Liu–Wei–Di–Huang–Wan in type 2 diabetes mellitus mice. Int J Anal Chem 2020:1306439
Canfora EE, Meex RCR, Venema K, Blaak EE (2019) Gut microbial metabolites in obesity, NAFLD and T2DM. Nat Rev Endocrinol 15(5):261–273
Parada Venegas D, De la Fuente MK, Landskron G et al (2019) Short Chain Fatty Acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol 10:277
Mandaliya DK, Seshadri S (2019) Short chain fatty acids, pancreatic dysfunction and type 2 diabetes. Pancreatology 19(2):280–284
Michalowska J, Miller-Kasprzak E, Bogdanski P (2021) Incretin hormones in obesity and related cardiometabolic disorders: the clinical perspective. Nutrients 13(2):66
Gribble FM, Reimann F (2021) Metabolic messengers: glucagon-like peptide 1. Nat Metab 3(2):142–148
Miguens-Gomez A, Casanova-Marti A, Blay MT et al (2021) Glucagon-like peptide-1 regulation by food proteins and protein hydrolysates. Nutr Res Rev 34(2):259–275
Tolhurst G, Heffron H, Lam YS et al (2012) Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 61(2):364–371
Huang JH, He D, Chen L et al (2020) A GC–MS-based metabolomics investigation of the protective effect of Liu–Wei–Di–Huang–Wan in type 2 diabetes mellitus mice. Int J Anal Chem 2020:1306439
Peng W, Huang J, Yang J et al (2019) Integrated 16S rRNA sequencing, metagenomics, and metabolomics to characterize gut microbial composition, function, and fecal metabolic phenotype in non-obese type 2 diabetic Goto–Kakizaki rats. Front Microbiol 10:3141
Estaki M, Jiang L, Bokulich NA et al (2020) QIIME 2 enables comprehensive end-to-end analysis of diverse microbiome data and comparative studies with publicly available data. Curr Protoc Bioinform 70(1):e100
Segata N, Izard J, Waldron L et al (2011) Metagenomic biomarker discovery and explanation. Genome Biol 12(6):R60
Douglas GM, Maffei VJ, Zaneveld JR et al (2020) PICRUSt2 for prediction of metagenome functions. Nat Biotechnol 38(6):685–688
Caspi R, Billington R, Keseler IM et al (2020) The MetaCyc database of metabolic pathways and enzymes—a 2019 update. Nucleic Acids Res 48(D1):D445–D453
Magliano DJ, Sacre JW, Harding JL et al (2020) Young-onset type 2 diabetes mellitus—implications for morbidity and mortality. Nat Rev Endocrinol 16(6):321–331
Sebastian Domingo JJ, Sanchez SC (2018) From the intestinal flora to the microbiome. Rev Esp Enferm Dig 110(1):51–56
Perreault L, Skyler JS, Rosenstock J (2021) Novel therapies with precision mechanisms for type 2 diabetes mellitus. Nat Rev Endocrinol 17(6):364–377
Xu L, Li Y, Dai Y, Peng J (2018) Natural products for the treatment of type 2 diabetes mellitus: pharmacology and mechanisms. Pharmacol Res 130:451–465
Wang J, Ma Q, Li Y et al (2020) Research progress on Traditional Chinese Medicine syndromes of diabetes mellitus. Biomed Pharmacother 121:109565
Guest PC (2019) Characterization of the Goto–Kakizaki (GK) rat model of type 2 diabetes. Methods Mol Biol 1916:203–211
Dai B, Wu Q, Zeng C et al (2016) The effect of Liuwei Dihuang decoction on PI3K/Akt signaling pathway in liver of type 2 diabetes mellitus (T2DM) rats with insulin resistance. J Ethnopharmacol 192:382–389
Giongo A, Gano KA, Crabb DB et al (2011) Toward defining the autoimmune microbiome for type 1 diabetes. ISME J 5(1):82–91
Yan F, Li N, Shi J et al (2019) Lactobacillus acidophilus alleviates type 2 diabetes by regulating hepatic glucose, lipid metabolism and gut microbiota in mice. Food Funct 10(9):5804–5815
Vallianou NG, Stratigou T, Tsagarakis S (2018) Microbiome and diabetes: Where are we now? Diabetes Res Clin Pract 146:111–118
Horton F, Wright J, Smith L, Hinton PJ, Robertson MD (2014) Increased intestinal permeability to oral chromium (51 Cr) -EDTA in human type 2 diabetes. Diabet Med 31(5):559–563
Ding S, Lund PK (2011) Role of intestinal inflammation as an early event in obesity and insulin resistance. Curr Opin Clin Nutr Metab Care 14(4):328–333
Forslund K, Hildebrand F, Nielsen T et al (2015) Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature 528(7581):262–266
Zhao JD, Li Y, Sun M et al (2021) Effect of berberine on hyperglycaemia and gut microbiota composition in type 2 diabetic Goto-Kakizaki rats. World J Gastroenterol 27(8):708–724
Ren SM, Mei L, Huang H et al (2019) Correlation analysis of gut microbiota and biochemical indexes in patients with non-alcoholic fatty liver disease. Zhonghua Gan Zang Bing Za Zhi 27(5):369–375
Pedersen HK, Gudmundsdottir V, Nielsen HB et al (2016) Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 535(7612):376–381
Cani PD, de Vos WM (2017) Next-generation beneficial microbes: the case of Akkermansia muciniphila. Front Microbiol 8:1765
Everard A, Belzer C, Geurts L et al (2013) Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci USA 110(22):9066–9071
Zhai Q, Feng S, Arjan N, Chen W (2019) A next generation probiotic, Akkermansia muciniphila. Crit Rev Food Sci Nutr 59(19):3227–3236
Gaspar P, Neves AR, Ramos A et al (2004) Engineering Lactococcus lactis for production of mannitol: high yields from food-grade strains deficient in lactate dehydrogenase and the mannitol transport system. Appl Environ Microbiol 70(3):1466–1474
Xie M, Chen HH, Nie SP, Yin JY, Xie MY (2017) Gamma-aminobutyric acid increases the production of short-chain fatty acids and decreases pH values in mouse colon. Molecules 22(4):66
Kim M, Qie Y, Park J, Kim CH (2016) Gut microbial metabolites fuel host antibody responses. Cell Host Microbe 20(2):202–214
Patra KC, Hay N (2014) The pentose phosphate pathway and cancer. Trends Biochem Sci 39(8):347–354
Basen M, Kurrer SE (2021) A close look at pentose metabolism of gut bacteria. Febs J 288(6):1804–1808
Hernandez MAG, Canfora EE, Jocken JWE, Blaak EE (2019) The short-chain fatty acid acetate in body weight control and insulin sensitivity. Nutrients 11(8):66
Kim CH, Park J, Kim M (2014) Gut microbiota-derived short-chain fatty acids, T cells, and inflammation. Immune Netw 14(6):277–288
Tedelind S, Westberg F, Kjerrulf M, Vidal A (2007) Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: a study with relevance to inflammatory bowel disease. World J Gastroenterol 13(20):2826–2832
O’Sullivan JF, Morningstar JE, Yang Q et al (2017) Dimethylguanidino valeric acid is a marker of liver fat and predicts diabetes. J Clin Invest 127(12):4394–4402
Oh NS, Lee JY, Kim YT, Kim SH, Lee JH (2020) Cancer-protective effect of a synbiotic combination between Lactobacillus gasseri 505 and a Cudrania tricuspidata leaf extract on colitis-associated colorectal cancer. Gut Microbes 12(1):1785803
Zheng F, Wang Z, Stanton C et al (2021) Lactobacillus rhamnosus FJSYC4-1 and Lactobacillus reuteri FGSZY33L6 alleviate metabolic syndrome via gut microbiota regulation. Food Funct 12(9):3919–3930
Yu L, Han X, Cen S et al (2020) Beneficial effect of GABA-rich fermented milk on insomnia involving regulation of gut microbiota. Microbiol Res 233:126409
Ze X, Duncan SH, Louis P, Flint HJ (2012) Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. ISME J 6(8):1535–1543
Mueller NT, Differding MK, Zhang M et al (2021) Metformin affects gut microbiome composition and function and circulating short-chain fatty acids: a randomized trial. Diabetes Care 44(7):1462–1471
Nauck MA, Meier JJ (2018) Incretin hormones: their role in health and disease. Diabetes Obes Metab 20(Suppl 1):5–21
Stemmer K, Finan B, DiMarchi RD, Tschöp MH, Müller TD (2020) Insights into incretin-based therapies for treatment of diabetic dyslipidemia. Adv Drug Deliv Rev 159:34–53
Dominguez-Bello MG, Godoy-Vitorino F, Knight R, Blaser MJ (2019) Role of the microbiome in human development. Gut 68(6):1108–1114
Hermes-Uliana C, Frez FCV, Sehaber CC et al (2018) Supplementation with l-glutathione improves oxidative status and reduces protein nitration in myenteric neurons in the jejunum in diabetic Rattus norvegicus. Exp Mol Pathol 104(3):227–234
Genser L, Aguanno D, Soula HA et al (2018) Increased jejunal permeability in human obesity is revealed by a lipid challenge and is linked to inflammation and type 2 diabetes. J Pathol 246(2):217–230
Acknowledgements
This work was supported by the National Key R&D Program of China (No:2018YFC1704400); National Nature Science Foundation of China (No. 82074400, U21A20411); Natural Science Foundation of Hunan Province (2020JJ5325); Key Research and Development Project of Hunan Province Science and Technology (2020ZYQ038, 2020SK2029, 2020SK2101); Project of Changsha Technology Innovation Center (kh2004018); Training Program for Excellent Young Innovators of Changsha (kq1802017); Research Project of Traditional Chinese Medicine Bureau of Hunan Province (202092); Program of Survey of Chinese Medicines of China ([2017]66), Research on the Comprehensive Development and Utilization of Characteristic Traditional Chinese Medicine Resources (2060302). China Post-doctoral Science Foundation (2022M711131)
Author information
Authors and Affiliations
Contributions
DH, YW designed the experiments, supervised, and participated in the entire work. ZYY, LC were in charge of statistical analysis and wrote the manuscript and revised the manuscript. DZ maintained and performed animal studies. SHZ participated in study design and data collection. All the authors revised and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Ethics statement
The animal study was reviewed and approved by The Animal Ethical Committee of Hunan Academy of Chinese Medicine.
Research Involving Human and Animal Rights
Our study did not involve human studies.
Informed consent
Informed written consent was obtained from the participants before all the study procedures.
Additional information
Managed by Massimo Federici.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yi, Zy., Chen, L., Wang, Y. et al. The potential mechanism of Liu–Wei–Di–Huang Pills in treatment of type 2 diabetic mellitus: from gut microbiota to short-chain fatty acids metabolism. Acta Diabetol 59, 1295–1308 (2022). https://doi.org/10.1007/s00592-022-01922-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-022-01922-y