Abstract
Aim
Management of diabetes care can be affected by COVID-19 pandemic control measures. This study aimed to determine the impact of the pandemic, during 17.03.2020–16.03.2021, on quality outcomes of diabetes care in general practice in Switzerland.
Methods
In this retrospective cohort study, diabetes mellitus patients (≥ 18 years) with at least one consultation at a general practitioner, during 17.03.2018–16.03.2019 (cohort 1) and 17.03.2019–16.03.2020 (cohort 2) were included and followed-up for two years. Quality indicators and outcomes of diabetes care, at patient and practitioner level, were compared before and during the pandemic. Logistic regression was performed to identify patient’s risk factors for dropout from follow-up.
Results
Data from 191 practices, 23,903 patients, cohort 1 and 25,092 patients, cohort 2, were analyzed. The fraction of patients lost to follow-up, attributable to the pandemic, was 28% (95% confidence interval: 25%, 30%). During the pandemic, compared to the previous year, regular measurement of weight, HbA1c, blood pressure and serum creatinine were less frequent and less patients per practitioner reached HbA1c and blood pressure target outcomes. Factors associated with continuity of care during the pandemic were: patient age 41–80 years, longer diabetes duration, diagnosis of hypertension or dyslipidemia, influenza vaccination during the last year. Risk factors for dropout were age > 80 and receiving only insulin as anti-diabetic medication.
Conclusion
A considerable quality reduction in diabetes mellitus care could be observed during the pandemic. Though the most vulnerable patients were not the most affected by the pandemic, key factors that might reduce dropout from follow-up were identified.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During SARS-CoV-2 pandemic, management of chronic non-communicable diseases (NCDs) such as diabetes, hypertension, dyslipidemia can be affected in several ways. Even in the absence of an overload of COVID-19 cases, disease control measures, such as lockdown, quarantine, restrictions of public and private transport or fear of infection might have an impact on accessibility of health care [1]. In Switzerland, non-urgent patient care was suspended during lockdown (17.03.2020–26.04.2020) [2].
Several studies have examined short-term effects of COVID-19 pandemic, during or after lockdown, on glycaemic control [3,4,5,6,7,8,9,10,11,12,13,14,15,16].
Impact of COVID-19 pandemic on quality of diabetes care, as promoted through the Quality and Outcomes Framework (QOF) [17, 18], has been scarcely measured [19]. Processes and outcome indicators for type 2 diabetes patients, according to the Italian guidelines, were also compared between 2019 and 2020 [20].
This study aimed, first, to assess the impact of SARS-CoV-2 pandemic, during 17.03.2020–16.03.2021, on quality indicators and outcomes of diabetes care, based on [17, 18] and adapted for primary care in Switzerland [21,22,23]; second, to identify factors associated with patient dropout from follow-up during the pandemic.
Methods
Study design and population
This retrospective cohort study used the database from FIRE project (Family Medicine ICPC (International Classification of Primary Care) Research Using Electronic Medical Records) [24, 25] which acquires medical data from general practitioners in Switzerland.
Adult patients (≥ 18 years) with diabetes mellitus and at least one encounter at a general practitioner during baseline year: 17.03.2018–16.03.2019, cohort 1, and 17.03.2019–16.03.2020, cohort 2 (pandemic-exposed), were included and followed-up respectively before the Swiss lockdown, until 16.03.2020, and from Swiss lockdown until one year, 16.03.2021.
Diabetes mellitus diagnosis was based on one of the following conditions from whole patient history: i) at least two measurements HbA1c ≥ 6.5% (48 mmol/mol), as recommended [26]; ii) prescription of any anti-diabetic medication (Anatomical Therapeutic Chemical Classification System (ATC) [27] code A10); iii) International Classification of Primary Care 2nd edition (ICPC-2) [28] diagnosis Code T89 or T90. As Glucagon-Like-Peptide (GLP)-1 receptor agonists and Sodium-dependent Glucose Transporter 2 (SGLT-2) inhibitors could be prescribed for other reasons than diabetes (obesity and congestive heart failure or chronic kidney disease, respectively) patients treated exclusively with them were included if in addition at least once HbA1c ≥ 6.5% (48 mmol/mol).
Data description
Patient age, in years, was defined both as continuous and categorical variable (≤ 40, 41–60, 61–80, > 80 years).
Postal code of the physician practice was used to identify urban, suburban, rural areas [29].
Comorbidities were identified through ATC codes, Global Trade Item Number (GTIN), Pharmaceutical cost groups (PCG) [30], ICPC-2 diagnosis codes [28] or laboratory measurements; details in Online Resource 1 Table 1.
Time from first diabetes diagnosis to study start was categorized as: first diagnosis during baseline, first diagnosis < 1 year, 1–5 years and > 5 years.
Insulin-dependent, non-insulin dependent and unknown was a proxy for diabetes type (Online Resource 1 Table 2).
Single diabetes medications were: Metformin, Sulfonylurea, Dipeptidylpeptidase (DPP)-4 inhibitors, SGLT-2 inhibitors, GLP-1 receptor agonists, basal insulin therapy, basal-bolus insulin therapy and other. Mixed or combination of two or more therapies were counted separately in each medication. Anti-diabetic medications were also grouped: insulin only, insulin plus oral-anti-diabetic therapy, oral-anti-diabetic (OAD) monotherapy and OAD combination therapy (Online Resource 1 Table 2).
Other medications relevant to diabetes care were: Aspirin, statins, RAAS-inhibitors. Data on influenza vaccination was also reported (Online Resource 1 Table 3).
New or not expired prescriptions, during the observation period, were included. For prescriptions without defined stop dates, a validity of 365 days was supposed, as most of diabetes patients with a particular treatment, had the same prescription in the following 12 months [31].
Indicators of diabetes care quality [21,22,23] were defined as proportions of patients with the following outcomes in a year interval: (1) at least two HbA1c measurements; (2) average HbA1c ≤ 7.0% (53 mmol/mol); (3) average HbA1c ≤ 8.0% (64 mmol/mol); (4) average HbA1c ≤ 9.0% (75 mmol/mol); (5) at least two blood pressure measurements; (6) average blood pressure < 140/90 mmHg; (7) at least one low density lipoprotein (LDL)-cholesterol measurement; (8) average LDL-cholesterol < 2.6 mmol/l; (9) at least one weight or body mass index (BMI) measurement; (10) at least one serum creatinine and microalbuminuria measurement.
Statistical analysis
Baseline characteristics of each cohort: during 17.03.2018–16.03.2019 and 17.03.2019–16.03.2020 respectively, were described as number and percentage, N (%), for categorical or binary variables and as mean standard deviation (SD) for continuous variables. χ2-test, for categorical or binary variables, or t-test, for continuous variables, were performed for cohort comparisons or baseline and follow-up comparisons within cohorts.
Trends of all HbA1c values, weekly averaged over patients by cohort, were shown graphically.
For each cohort, from baseline to follow-up, differences between average values, for laboratory measurements, or between proportions, for quality indicators, were reported with 95% confidence interval (CI).
A subgroup analysis of outcome indicators during each year, for patients included in both cohorts, was also performed.
Quality indicator results were shown through a dumbbell plot or connected dot plot. At practice level, the median patient proportion, in each cohort, for each indicator, during baseline and follow-up periods, was reported with the interquartile range [IQR] and represented through error bar plots.
Population attributable fraction (PAF) [32] was used to compare proportions of cases in the two cohorts, with complementary outcome (dropout from follow-up, not reaching quality target …) during follow-up, considering cohort 2 being pandemic-exposed. PAF was shown graphically through a bar chart with 95% (CI) error bars.
To identify risk factors for dropout from follow-up during the first year of pandemic, for cohort 2, unadjusted and multivariable-adjusted mixed logistic regression models were performed. Random effects were considered at practice level, to correct for correlation between patients followed by the same practice. Predictors in multivariable analysis were selected with a stepwise backward approach, starting from a full model including all variables, not correlated among them, with p < 0.2 in univariable analysis. Results of regression analysis were reported as odds ratio (OR) (95%(CI)). Multivariable analysis results were represented through an odds ratio plot.
For all tests, p ≤ 0.05 was considered statistically significant. All analyses were carried out using statistical package R version 4.1.0 [33].
Results
Patient characteristics
A total of 27,043 patients and 191 practices were included: cohort 1, baseline 17.03.2018–16.03.2019, 23,903 patients; cohort 2, baseline 17.03.2019–16.03.2020, 25,092 patients; 21,952 patients in both cohorts, Fig. 1.
Female proportion was 43% in each cohort, p = 0.73. Age was 65.45(14.89) years, cohort 1, and 65.33(14.63), cohort 2, p = 0.38, Table 1.
First diagnosis of diabetes mellitus occurred during baseline in 8223(34%) patients, cohort 1; 9667(39%) patients, cohort 2, p < 0.001.
Hypertension was the most prevalent single comorbidity: 13,221(55%) cohort 1; 14,608(58%) cohort 2, p < 0.001, followed by dyslipidemia, obesity, and cardiovascular diseases (such as coronary heart disease, stroke).
Anti-diabetic and other medication prescriptions
The most prevalent therapy was OAD monotherapy: 11,760(49%) in cohort 1 and 14,334(57%) in cohort 2, p < 0.001 and metformin was the most used OAD: 11,868(50%), cohort 1 and 14,321(57%), cohort 2, p < 0.001.
Insulin-dependent patients were 4274(18%) in cohort 1, 5140(21%) in cohort 2.
Less patients had no anti-diabetic medication and no medication at all in cohort 2 compared to cohort 1: 7248(29%) versus 8946(37%), p < 0.001, Table 1; 3613(14%) versus 4912(20%), p < 0.001, Online Resource 1 Table 4.
During cohort 2 follow-up, medications prevalence was higher compared to baseline, but PAF was significant only for SGLT-2 and GLP-1 (9% and 6%), Online Resource 1 Table 5.
Quality indicators (patient level)
In cohort 1, 1951(8%) patients were lost to follow-up during 17.03.2019–16.03.2020; in cohort 2, 3598(14%) during 17.03.2020–16.03.2021; PAF 28%(25, 30)%, Fig. 2. Youngest patients, age ≤ 40 years, had the greatest PAF for dropout, 37%(30, 43)%; oldest patients, age > 80, had the lowest PAF 19%(13, 24)%, Online Resource 1 Table 5.
Evolution, from baseline to follow-up year, of quality indicators at patient level and impact of COVID-19 pandemic. Proportions of patients, in each year for each cohort, were represented through connected dots. Differences with 95% confidence interval (CI) were reported in columns. Attributable fraction in the exposed (cohort 2) and in the population, PAF (two cohorts), the latter with 95% confidence interval (CI), were reported in the barplot (right side). They were calculated, for the complementary outcome of each indicator in the follow-up year, as difference between the respective proportion and the proportion of unexposed cases in cohort 1. In legend, pandemic meant attributable to the pandemic exposure. Abbreviations: n: number of reported measurements per patient; HbA1c: Hemoglobin A1c; BP: blood pressure; LDL: low density lipoprotein
In cohort 1, 12,939(54%) had weight recorded during follow-up versus 13,332(56%) during baseline, difference − 2.0%(− 2.5, − 0.7)%, Fig. 2. In cohort 2, the difference was − 10%(− 11.2, − 9.5)%. PAF was 6%(5, 7)%. Average weight did not change during follow-up for each cohort, Online Resource 1 Table 6.
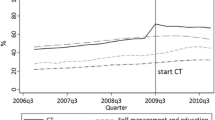
The proportion of patients, cohort 1, with HbA1c recorded, decreased by 4.5%(4.0, 5.0)%, absolute difference, during follow-up, starting from 78%, Fig. 2. In cohort 2, the difference was − 17.6%(− 18.0, − 17.0)% starting from 79%. PAF was 19%(18, 20)%. Average HbA1c reduced, − 0.04% (0.4 mmol/mol) difference, during follow-up in each cohort, Online Resource 1 Table 6. Weekly averages HbA1c during follow-up were higher in cohort 2, compared to cohort 1, from June 2020 to November 2020, Fig. 3.
Evolution of Hemoglobin A1c (HbA1c) % values, from baseline to follow-up year for each cohort. HbA1c % were weekly averaged over patients. Points represented observed values and lines the smoothed curves. Dashed lines marked the period from 17.03.2020 to 26.04.2020, the national lockdown in Switzerland
Blood pressure was reported, during baseline, in 17,614(74%) patients in cohort 1 with a − 8.0% (− 8.8, − 7.2)% difference during follow-up; for cohort 2, the difference was − 16.6%(− 17.4, − 15.8)%, Fig. 2. PAF was 14%(13, 15)%.
As only a minority of patients had LDL-cholesterol recorded, PAF was not evaluated. However, average LDL-cholesterol improved during follow-up in each cohort, Online Resource 1 Table 6.
Patients with serum creatinine recorded during follow-up decreased more in cohort 2, than in cohort 1, Fig. 2. PAF was 5%(4, 6)%.
Since microalbuminuria was scarcely reported PAF was not evaluated.
For most indicators, around one third of patients included in both cohorts reached outcome indicator during the pre-pandemic (17.03.2018–16.03.2020) but not in the pandemic year and around one fifth of patients never reached outcome indicator during 17.03.2018–16.03.2021, Online Resource 1 Table 7.
Quality indicators (practice level)
During baseline, in half the practices 76% of cohort 1 and 77% of cohort 2 patients had an average HbA1c ≤ 9.0% (75 mmol/mol); during follow-up, 80% of cohort 1 and 76% of cohort 2, Fig. 4. Of all patients with average HbA1c > 9.0% (75 mmol/mol) during follow-up, 16%(15, 17)% was the pandemic-attributable fraction. Similar numbers resulted in other HbA1c indicators but with lower PAF: 6% (5.7, 7)%, HbA1c > 7% (53 mmol/mol); 12%(11, 13)%, HbA1c > 8% (64 mmol/mol).
Evolution, from baseline to follow-up year, of quality indicators at practice level (Swiss Quality and Outcome Framework) and impact of COVID-19 pandemic. Median, at practice level, of the percentage of patients, for each cohort who fulfilled the indicator was reported in the error bar with the interquartile range [IQR]. Dashed line represented the quality reference area, or threshold, for each indicator. Attributable fraction in the exposed (cohort 2) and in the population, PAF (two cohorts), the latter with 95% confidence interval (CI), were reported in the barplot (right side). They were calculated, for the complementary outcome of each indicator in the follow-up year, as difference between the respective proportion and the proportion of unexposed cases in cohort 1. In legend, pandemic meant attributable to the pandemic exposure. Abbreviations: n: number of reported measurements per patient; HbA1c: Hemoglobin A1c; BP: blood pressure; LDL: low density lipoprotein
During baseline, in half the practices, 49% of cohort 1 and 50% of cohort 2 had blood pressure recorded at least twice; during follow-up, 52% of cohort 1 and 43% of cohort 2. PAF was 7%(6, 8)%. In half the practices, 40% of the patients, of each cohort, had an average blood pressure < 140/90 mmHg, baseline period. During follow-up, the median was 41% of cohort 1 and 33% of cohort 2. PAF was 6%(5, 6.2)%.
LDL quality indicators improved, from baseline to follow-up in each cohort, though far from the ideal threshold (dashed line). As most patients did not reach outcome targets, PAF for these outcomes were not evaluated.
Factors associated with dropout from follow-up during the pandemic
Online Resource 1 Table 8 and Fig. 5 reported results of univariable and multivariable analysis of factors associated with dropout from follow-up during the pandemic. From multivariable analysis, protective factors against dropout were: patient age 41–60, OR (95% CI), 0.71(0.59, 0.86) p < 0.001; age 61–80 0.76(0.63, 0.92) p = 0.004; time from diabetes diagnosis > 5 years 0.76(0.62, 0.92), p = 0.005; diagnosis of hypertension 0.68(0.61, 0.75), p < 0.001; diagnosis of dyslipidemia 0.68(0.61, 0.75), p < 0.001; influenza vaccination in previous year 0.30(0.25, 0.37), p < 0.001. Risk factors for dropout were: age > 80 1.49(1.22, 1.82), p < 0.001; receiving only insulin as anti-diabetic medication 1.59(1.36, 1.87), p < 0.001.
Factors associated with dropout during COVID-19 pandemic. Odds ratio (OR) plot with 95% confidence interval (CI). Multivariable mixed logistic regression analysis performed with practice as random effect. Data of cohort 2 were considered with 25,092 patients and 191 practices. Predictors were considered in only one period, 17.03.2019-16.03.2020: one value per patient
Discussion
Summary
In this study, the impact of COVID-19 pandemic on quality and outcomes of diabetes care was evaluated. The main findings are: (i) 28% of total dropout from follow-up during the observation period was attributable to the pandemic; (ii) the proportion of patients with regular measurement of weight, HbA1c, blood pressure and serum creatinine decreased during the pandemic compared to the previous year; (iii) at practice level, the proportion of patients reaching HbA1c and blood pressure target decreased during the pandemic compared to the previous year; (iv) factors associated with continued care during the pandemic were: patient age 41–80; longer diabetes onset; diagnosis of hypertension or dyslipidemia; influenza vaccination in the previous year. Risk factors for dropout from follow-up were age > 80 and receiving only insulin as anti-diabetic medication.
Strengths and limitations
A strength of this study is the large database used whose validity is also supported, being the distribution of age, gender, as well as prescription proportions of anti-diabetic medications in agreement with Swiss health care settings [4, 34].
Conversely, there are some limitations. First, data quality had impact on baseline characteristics differences between the two cohorts. Moreover, we could not reliably distinguish between types 1 and type 2 patients or even be sure to have included all diabetes patients. Second, the database used (FIRE) only includes general practitioners but some of them could have a double specialty in endocrinology and general internal medicine. Moreover, some patients might have been followed by general practitioners and endocrinologists at the same time, having made their laboratory analyses at the general practitioner, but received their prescription at the endocrinologist, or vice versa. Third, we had no information about patient adherence or tolerance to treatment that could have influenced prescribing decisions. Fourth, data concerning patient-physician contact (face-to-face consultation, telephone or video-call) was unrecorded. Fifth, reason for dropout from follow-up (death, hospitalization/institutionalization, change of physician) was unknown. Sixth, prescriptions without defined stop dates might have been overestimated, supposing a validity of 365 days. Seventh, we could not examine the impact of socio-economic or life-style variables. Last, information about COVID testing or infection was missing. This could have affected dropout, increased comorbidities during the pandemic, glycaemic control or prescriptions.
Comparison with existing literature
Pandemic and dropout from follow-up
During 17.03.2020–16.03.2021 the dropout rate was 14.3% of which 43% attributable to the pandemic. At population level, the pandemic-attributable fraction was 28%. Several studies found a negative impact of lockdown on consultations for diabetes patients: weekly consultations were 17.5% lower than expected without lockdown [35]; during lockdown 49% of patients did not consult their general practitioners [3], similar to [36, 37], while in India the majority reported no access to healthcare services [38]. In a large cohort of around 250′000 type 2 diabetes patients in Italy, an overall reduction of 24% in follow-up visits was observed during 2020, compared to 2019 [20]. As no study evaluated the proportion of dropout cases attributable to the pandemic, in a year time after lockdown started, our findings are not directly comparable with the existing literature.
Pandemic and anti-diabetic prescriptions
During the pandemic and compared to the previous year, the proportion of patients with medications increased in our study and for SGLT-2 and GLP-1 a significant effect was attributable to the pandemic. Other studies observed: an increase in insulin [39] and both insulin and OAD medications [40, 41], during the first month of pandemic as compared to the year before; a decrease in OAD during the first four months of the pandemic [42].
Pandemic and quality indicators of diabetes care
According to our findings, the reduction in measurement counts, for all primary care patients, was more pronounced than the reduction in consultation counts [35], though our results were at patient level and not at consultation or measurement counts. Marked reductions in the rate of health checks of type 2 diabetes patients, between March and December 2020, were highlighted [19, 20]. Accordingly, quality at patient level declined, during the pandemic, in particular for the number of patients with HbA1c recorded (17.6% absolute difference) and for the number of patients with blood pressure recorded (16.6% absolute different). However, differently from the literature, we were the first reporting the pandemic-attributable fraction for not having HbA1c and blood pressure records: PAF 19% and 14%, lesser than the one for dropout from follow-up, 28%.
Results of glycaemic decompensation, during lockdown or after few months, are conflicting [13]: no differences [3,4,5]; worsening [6,7,8, 14, 15]; improving [9,10,11,12].
We considered a larger time frame compared to these studies. Being HbA1c the most important variable of diabetes care, we analyzed weekly averaged measurements during baseline and follow-up, by cohort, evidencing around 0.1% (1.1 mmol/mol) higher values, one month after the lockdown. That means higher glucose levels during lockdown, as HbA1c correlates with mean glucose level in the previous 8–12 weeks [43], though with minor clinical impact, as after five months HbA1c returned to the previous year's level.
Factors associated with dropout from follow-up during pandemic
To our knowledge, this is the first study to assess association of diabetes patient characteristics and medications with dropout from follow-up during a year from the lockdown. Reduction in follow-up visits in type 2 diabetes patients was independent of age, sex, and educational level [20]. Other studies investigated the reasons to avoid or postponed the visit during lockdown [3, 40]. Differently from [20], we found that dropout of youngest patients, age ≤ 40 years, was the most affected by the pandemic, PAF 37%(30, 43)% though the oldest ones, age > 80 years, had the highest risk of dropout, after correcting for confounders. However, this higher risk was not attributable to the pandemic, since dropout of oldest patients was the less affected, PAF 19%(13, 24)%, in line with [35, 44]. Therefore, since comorbidities were associated with regular care, the most vulnerable patients remained the main focus of primary care despite the pandemic.
Implications for research and/or practice
This study showed a decline in diabetes mellitus quality care during COVID-19 pandemic between 17.03.2020–16.03.2021, especially when facing HbA1c ≤ 7.0% (53 mmol/mol) and blood pressure < 140/90 mmHg. For most indicators, around one third of patients, included in both cohorts, reached the quality outcome during the pre-pandemic years but no during the pandemic. However, there was also a relevant proportion of patients, around one fifth for most indicators, not reaching outcome indicator in every year of observation, suggesting room for improvement in quality of diabetes care, independently of the pandemic. Though the most vulnerable patients (old, with more comorbidities) were not the most affected patients by the pandemic, our finding suggests key factors that might reduce dropout from follow-up of patients with diabetes mellitus. Primary care should have a primary role in guaranteeing continuity of care of these patients in order to prevent long-term adverse effects of the pandemic on diabetes complications.
Data availability
The datasets analyzed during the current study is available from the corresponding author on reasonable request.
References
Palmer K, Monaco A, Kivipelto M et al (2020) The potential long-term impact of the COVID-19 outbreak on patients with non-communicable diseases in Europe: consequences for healthy ageing. Aging Clin Exp Res 32(7):1189–1194. https://doi.org/10.1007/s40520-020-01601-4
The Swiss Federal Council (2020). COVID-19 Ordinance 3. Classified Compilation of Federal Law. https://www.admin.ch/opc/en/classified-compilation/20200744/index.html. Accessed 2 June 2020
Ludwig L, Scheyer N, Remen T, Guerci B (2021) The impact of COVID-19 lockdown on metabolic control and access to healthcare in people with diabetes: the CONFI-DIAB cross-sectional study. Diabetes Ther 12(8):2207–2221. https://doi.org/10.1007/s13300-021-01105-y
Zechmann S, Hotz L, Di Gangi S, Baumgartl K, Plate A, Potlukova E (2021) Impact of SARS-CoV-2 lockdown on glycaemic control: a retrospective observational cohort study in a tertiary setting. J Clin Med 10(18):4098. https://doi.org/10.3390/jcm10184098
Silverii GA, Delli Poggi C, Dicembrini I, Monami M, Mannucci E (2021). Glucose control in diabetes during home confinement for the first pandemic wave of COVID-19: a meta-analysis of observational studies. Acta Diabetol. 58(12):1603-1611. https://doi.org/10.1007/s00592-021-01754-2. Epub 2021. Erratum in: Acta Diabetol. 2021 Aug 2
Biancalana E, Parolini F, Mengozzi A, Solini A (2021) Short-term impact of COVID-19 lockdown on metabolic control of patients with well-controlled type 2 diabetes: a single-centre observational study. Acta Diabetol 58(4):431–436. https://doi.org/10.1007/s00592-020-01637-y
Önmez A, Gamsızkan Z, Özdemir Ş et al (2020) The effect of COVID-19 lockdown on glycemic control in patients with type 2 diabetes mellitus in Turkey. Diabetes Metab Syndr 14(6):1963–1966. https://doi.org/10.1016/j.dsx.2020.10.007
Karatas S, Yesim T, Beysel S (2021) Impact of lockdown COVID-19 on metabolic control in type 2 diabetes mellitus and healthy people. Prim Care Diabetes 15(3):424–427. https://doi.org/10.1016/j.pcd.2021.01.003
Bonora BM, Boscari F, Avogaro A, Bruttomesso D, Fadini GP (2020) Glycaemic control among people with type 1 diabetes during lockdown for the SARS-CoV-2 outbreak in Italy. Diabetes Ther 11(6):1–11. https://doi.org/10.1007/s13300-020-00829-7
Fernández E, Cortazar A, Bellido V (2020) Impact of COVID-19 lockdown on glycemic control in patients with type 1 diabetes. Diabetes Res Clin Pract 166:108348. https://doi.org/10.1016/j.diabres.2020.108348
Dover AR, Ritchie SA, McKnight JA et al (2021) Assessment of the effect of the COVID-19 lockdown on glycaemic control in people with type 1 diabetes using flash glucose monitoring. Diabet Med 38(1):e14374. https://doi.org/10.1111/dme.14374
Rastogi A, Hiteshi P, Bhansali A (2020) Improved glycemic control amongst people with long-standing diabetes during COVID-19 lockdown: a prospective, observational, nested cohort study. Int J Diabetes Dev Ctries. https://doi.org/10.1007/s13410-020-00880-x
Eberle C, Stichling S (2021) Impact of COVID-19 lockdown on glycemic control in patients with type 1 and type 2 diabetes mellitus: a systematic review. Diabetol Metab Syndr 13(1):95. https://doi.org/10.1186/s13098-021-00705-9
Hosomi Y, Munekawa C, Hashimoto Y et al (2022) The effect of COVID-19 pandemic on the lifestyle and glycemic control in patients with type 1 diabetes: a retrospective cohort study. Diabetol Int 13(1):85–90. https://doi.org/10.1007/s13340-021-00507-4
Verma A, Rajput R, Verma S, Balania VKB, Jangra B (2020) Impact of lockdown in COVID 19 on glycemic control in patients with type 1 diabetes mellitus. Diabetes Metab Syndr 14(5):1213–1216. https://doi.org/10.1016/j.dsx.2020.07.016
Park SD, Kim NY, Jeon JH et al (2021) Impact of urgently initiated tele-prescription due to COVID-19 on glycemic control in patients with type 2 diabetes. Korean J Intern Med 36(4):942–948. https://doi.org/10.3904/kjim.2020.464
Roland M (2004) Linking physicians’ pay to the quality of care–a major experiment in the United kingdom. N Engl J Med 351(14):1448–1454. https://doi.org/10.1056/NEJMhpr041294
NHS Digital (2020). Quality and Outcomes Framework 2019–20. https://digital.nhs.uk/data-and-information/publications/statistical/quality-and-outcomes-framework-achievement-prevalence-and-exceptions-data/2019-20#. Accessed 8 Dec 2020
Carr MJ, Wright AK, Leelarathna L et al (2021) Impact of COVID-19 restrictions on diabetes health checks and prescribing for people with type 2 diabetes: a UK-wide cohort study involving 618 161 people in primary care. BMJ Qual Saf. https://doi.org/10.1136/bmjqs-2021-013613
Giorda CB, Picariello R, Landriscina T et al (2022) Instructive lessons from the analysis of assistance in diabetes during the first phase of COVID-19 pandemic. Acta Diabetol 59(6):861–864. https://doi.org/10.1007/s00592-022-01855-6
Djalali S, Frei A, Tandjung R, Baltensperger A, Rosemann T (2014) Swiss quality and outcomes framework: quality indicators for diabetes management in Swiss primary care based on electronic medical records. Gerontology 60(3):263–273. https://doi.org/10.1159/000357370
Christ E, Brändle M, Czock A et al (2017). Kriterien für ein ‘gutes’ disease management diabetes in der Grundversorgung. The Swiss Society of Endocrinology and Diabetology. https://www.sgedssed.ch/fileadmin/user_upload/6_Diabetologie/64_Ressourcen_Hausarzt/Diabetes_Kriterien_2017_SGED_def.pdf. Accessed 8 Dec 2020
EQUAM (2020). Diabetes mellitus ─ Zertifizierte Behandlungsqualität - Programmbeschrieb mit den Indikatoren.https://www.equam.ch/wp-content/uploads/2020/06/33-Programmbeschrieb-Diabetes-d-V7.0.pdf. Accessed 8 Dec 2020
Chmiel C, Bhend H, Senn O, Zoller M, Rosemann T (2011) FIRE study-group. The FIRE project: a milestone for research in primary care in Switzerland. Swiss Med Wkly 140:w13142. https://doi.org/10.4414/smw.2011.13142
Das FIRE-Projekt. Institut für Hausarztmedizin, University of Zürich (2021). https://www.hausarztmedizin.uzh.ch/de/fire2.html. Accessed 4 Nov 2021
American Diabetes Association (2021). 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2021. Diabetes Care. 44(Suppl 1):S15-S33. https://doi.org/10.2337/dc21-S002. Erratum in: Diabetes Care. 2021;44(9):2182
WHO (2021). ATC/DDD Index. https://www.whocc.no/atc_ddd_index/. Accessed 14 Sept 2021
WHO. International Classification of Primary Care, Second edition (ICPC-2) 2020. Available at: https://www.who.int/classifications/icd/adaptations/icpc2/en/. Accessed 14 Sept 2021
Eurostat (2011). Degree of urbanisation (DEGURBA). Available at: https://ec.europa.eu/eurostat/web/degree-of-urbanisation/background. Accessed 30 May 2021
Bill M, Meyer D, Telser DH (2019). Aktualisierung der PCG-Liste für den Schweizer Risikoausgleich. Studie im Auftrag des Bundesamts für Gesundheit BAG. https://www.bag.admin.ch/dam/bag/de/dokumente/kuv-aufsicht/pus/risikoausgleich/corrigendun.pdf.download.pdf/Polynomics_Uni_Basel_Aktualisierung_PCG_Schlussbericht_2019-01-22.pdf. Accessed 30 May 2021
Bachmann KN, Roumie CL, Wiese AD et al (2020) Diabetes medication regimens and patient clinical characteristics in the national patient-centered clinical research network. PCORnet Pharmacol Res Perspect 8(5):e00637. https://doi.org/10.1002/prp2.637
Rosen L (2013) An intuitive approach to understanding the attributable fraction of disease due to a risk factor: the case of smoking. Int J Environ Res Public Health 10(7):2932–2943. https://doi.org/10.3390/ijerph10072932
R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed 30 May 2021
Kaiser A, Vollenweider P, Waeber G, Marques-Vidal P (2012) Prevalence, awareness and treatment of type 2 diabetes mellitus in Switzerland: the CoLaus study. Diabet Med 29(2):190–197. https://doi.org/10.1111/j.1464-5491.2011.03422.x
Rachamin Y, Senn O, Streit S, Dubois J, Deml MJ, Jungo KT (2021) Impact of the COVID-19 pandemic on the intensity of health services use in general practice: a retrospective cohort study. Int J Public Health 66:635508. https://doi.org/10.3389/ijph.2021.635508
Fisher L, Polonsky W, Asuni A, Jolly Y, Hessler D (2020) The early impact of the COVID-19 pandemic on adults with type 1 or type 2 diabetes: a national cohort study. J Diabetes Complic 34(12):107748. https://doi.org/10.1016/j.jdiacomp.2020.107748
Bonora BM, Morieri ML, Avogaro A, Fadini GP (2021) The Toll of Lockdown against COVID-19 on diabetes outpatient care: analysis from an outbreak area in Northeast Italy. Diabetes Care 44(1):e18–e21. https://doi.org/10.2337/dc20-1872
Khader MA, Jabeen T, Namoju R (2020) A cross sectional study reveals severe disruption in glycemic control in people with diabetes during and after lockdown in India. Diabetes Metab Syndr 14(6):1579–1584. https://doi.org/10.1016/j.dsx.2020.08.011
Frazer JS, Frazer GR (2021) Analysis of primary care prescription trends in England during the COVID-19 pandemic compared against a predictive model. Fam Med Community Health 9(3):e001143. https://doi.org/10.1136/fmch-2021-001143
Davin-Casalena B, Jardin M, Guerrera H et al (2021) The impact of the COVID-19 epidemic on primary care in South-eastern France: implementation of a real-time monitoring system based on regional health insurance system data. Rev Epidemiol Sante Publique 69(5):255–264. https://doi.org/10.1016/j.respe.2021.07.006
Engstrom T, Baliunas DO, Sly BP et al (2021) Toilet paper, minced meat and diabetes medicines: australian panic buying induced by COVID-19. Int J Environ Res Public Health 18(13):6954. https://doi.org/10.3390/ijerph18136954
Jacob L, Rickwood S, Rathmann W, Kostev K (2021) Change in glucose-lowering medication regimens in individuals with type 2 diabetes mellitus during the COVID-19 pandemic in Germany. Diabetes Obes Metab 23(4):910–915. https://doi.org/10.1111/dom.14293
Nathan DM, Turgeon H, Regan S (2007) Relationship between glycated haemoglobin levels and mean glucose levels over time. Diabetologia 50(11):2239–2244. https://doi.org/10.1007/s00125-007-0803-0
Joy M, McGagh D, Jones N et al (2020) Reorganisation of primary care for older adults during COVID-19: a cross-sectional database study in the UK. Br J Gen Pract 70(697):e540–e547. https://doi.org/10.3399/bjgp20X710933
Acknowledgements
The authors would like to thank the FIRE team for their expertise in data quality and support throughout clinical definitions, data extraction and data management. In particular, we would like to thank the Head of FIRE Jakob Martin Burgstaller MD DMD PhD, the Head of FIRE Research Stefan Markun MD and Levy Jäger MD, Giuseppe Pichierri PhD, Fabio Valeri Msc for technical support.
Funding
Open access funding provided by University of Zurich. The study received a research grant by KHM (Kollegium für Hausarztmedizin), https://khm-cmpr.ch/forschungsfonds-gewinner-2021/. The founder had no involvement in the study design; collection, analysis and data interpretation; writing of the report; or the decision to submit for publication.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Stefania Di Gangi. Funding acquisition was managed by Roland Fischer. Supervision was carried out by Andreas Zeller and Roland Fischer. The first draft of the manuscript was written by Stefania Di Gangi and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
As the project did not fall under the scope of the Federal Act on Research involving Human Beings (Human Research Act) (BASEC-Nr: Req-2017–0079), no ethical approval was needed.
Informed consent
As established by the Swiss Federal Law (HFG) and by the cantonal ethics committee, informed consent was not applicable because the study used anonymized data irreversibly altered, according also to the European General Data Protection Regulation.
Additional information
Managed by Massimo Porta.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Di Gangi, S., Lüthi, B., Diaz Hernandez, L. et al. Quality outcome of diabetes care during COVID-19 pandemic: a primary care cohort study. Acta Diabetol 59, 1189–1200 (2022). https://doi.org/10.1007/s00592-022-01920-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-022-01920-0