Abstract
Aim
Obesity and low-grade inflammation are associated with an increased risk of hepatocellular carcinoma (HCC), a leading cause of cancer-related death worldwide. The tissue inhibitor of metalloproteinase (TIMP) 3, an endogenous inhibitor of protease activity that represents a key mediator of inflammation, is reduced in inflammatory metabolic disorders and cancer. In contrast, Timp3-deficient mice (Timp3−/−) are highly resistant to developing HCC in response to a diethylnitrosamine (DEN); therefore, we aimed to elucidate the biological role of genetic loss of Timp3 in obesity-related hepatocarcinogenesis.
Methods
Fourteen-day-old male wild-type (wt) and Timp3−/− mice were injected with 25 mg/kg DEN or an equal volume of saline. After 4 weeks, mice were randomized into two dietary groups and fed either normal or high-fat diet and allowed to grow until 32 weeks of age. Liver histological features were analyzed, and differentially expressed genes in the liver were quantified.
Results
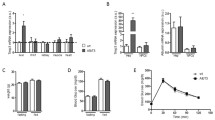
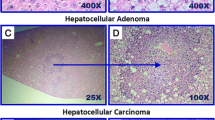
In Timp3−/− mice fed with the obesogenic diet, despite the increase in liver steatosis and inflammation, both the number of tumors and the total tumor size are significantly reduced 30 weeks post-DEN injection, compared to control mice. Moreover, Timp3 deletion in hepatocarcinogenesis during obesity is associated with a reduction in FoxM1 transcriptional activity through H19/miR-675/p53 pathway.
Conclusions
This study suggests that Timp3 ablation leads to cell cycle perturbation, at least in part by repressing FoxM1 transcriptional activity through H19/miR-675/p53 pathway.





Similar content being viewed by others
References
Agosti P, Sabbà C, Mazzocca A (2018) Emerging metabolic risk factors in hepatocellular carcinoma and their influence on the liver microenvironment. Biochim Biophys Acta Mol Basis Dis 1864:607–617. https://doi.org/10.1016/j.bbadis.2017.11.026
Lonardo A, Lugari S, Ballestri S, Nascimbeni F, Baldelli E, Maurantonio M (2019) A round trip from nonalcoholic fatty liver disease to diabetes: molecular targets to the rescue? Acta Diabetol 56:385–396. https://doi.org/10.1007/s00592-018-1266-0
Yang WS, Chen PC, Lin HJ et al (2017) Association between type 2 diabetes and cancer incidence in Taiwan: data from a prospective community-based cohort study. Acta Diabetol 54:455–461. https://doi.org/10.1007/s00592-017-0966-1
Federici M, Hribal ML, Menghini R et al (2005) Timp3 deficiency in insulin receptor haploinsufficient mice promotes diabetes and vascular inflammation via increased TNF-alpha. J Clin Invest 115:3494–3505. https://doi.org/10.1172/JCI26052
Menghini R, Menini S, Amoruso R et al (2009) Tissue inhibitor of metalloproteinase 3 deficiency causes hepatic steatosis and adipose tissue inflammation in mice. Gastroenterology 136:663–672.e4. https://doi.org/10.1053/j.gastro.2008.10.079
Menghini R, Fiorentino L, Casagrande V, Lauro R, Federici M (2013) The role of ADAM17 in metabolic inflammation. Atherosclerosis 228:12–17. https://doi.org/10.1016/j.atherosclerosis.2013.01.024
Fiorentino L, Vivanti A, Cavalera M et al (2010) Increased tumor necrosis factor alpha-converting enzyme activity induces insulin resistance and hepatosteatosis in mice. Hepatology 51:103–110. https://doi.org/10.1002/hep.23250
Anand-Apte B, Bao L, Smith R et al (1996) A review of tissue inhibitor of metalloproteinases-3(TIMP-3) and experimental analysis of its effect on primary tumor growth. Biochem Cell Biol 74:853–862
Kallio JP, Hopkins-Donaldson S, Baker AH, Kähäri VM (2011) TIMP-3 promotes apoptosis in non adherent small cell lung carcinoma cells lacking functional death receptor pathway. Int J Cancer 128:991–996. https://doi.org/10.1002/ijc.25404
Chavey C, Mari B, Monthouel MN et al (2003) Matrix metalloproteinases are differentially expressed in adipose tissue during obesity and modulate adipocyte differentiation. J Biol Chem 278:11888–11896. https://doi.org/10.1074/jbc.M209196200
Cardellini M, Menghini R, Martelli E et al (2009) TIMP3 is reduced in atherosclerotic plaques from subjects with type 2 diabetes and increased by SirT1. Diabetes 58:2396–2401. https://doi.org/10.2337/db09-0280
Lee JS, Chu IS, Mikaelyan A et al (2004) Application of comparative functional genomics to identify best-fit mouse models to study human cancer. Nat Genet 36:1306–1311. https://doi.org/10.1038/ng1481
Casagrande V, Mauriello A, Bischetti S, Mavilio M, Federici M, Menghini R (2017) Hepatocyte specific TIMP3 expression prevents diet dependent fatty liver disease and hepatocellular carcinoma. Sci Rep 7:6747. https://doi.org/10.1038/s41598-017-06439-x
Defamie V, Sanchez O, Murthy A, Khokha R (2015) TIMP3 controls cell fate to confer hepatocellular carcinoma resistance. Oncogene 34:4098–4108. https://doi.org/10.1038/onc.2014.339
Duan T, Sun W, Zhang M et al (2017) Dietary restriction protects against diethylnitrosamine-induced hepatocellular tumorigenesis by restoring the disturbed gene expression profile. Sci Rep 7:43745. https://doi.org/10.1038/srep43745
Rossi C, Marzano V, Consalvo A et al (2018) Proteomic and metabolomic characterization of streptozotocin-induced diabetic nephropathy in TIMP3-deficient mice. Acta Diabetol 55:121–129. https://doi.org/10.1007/s00592-017-1074-y
Menghini R, Casagrande V, Marino A et al (2014) MiR-216a: a link between endothelial dysfunction and autophagy. Cell Death Dis 5:e1029. https://doi.org/10.1038/cddis.2013.556
Hill-Baskin AE, Markiewski MM, Buchner DA et al (2009) Diet-induced hepatocellular carcinoma in genetically predisposed mice. Hum Mol Genet 18:2975–2988. https://doi.org/10.1093/hmg/ddp236
He D, Wang J, Zhang C et al (2015) Down-regulation of miR-675-5p contributes to tumor progression and development by targeting pro-tumorigenic GPR55 in non-small cell lung cancer. Mol Cancer 14:73. https://doi.org/10.1186/s12943-015-0342-0
Liu C, Chen Z, Fang J, Xu A, Zhang W, Wang Z (2016) H19-derived miR-675 contributes to bladder cancer cell proliferation by regulating p53 activation. Tumour Biol 37:263–270. https://doi.org/10.1007/s13277-015-3779-2
Engeland K (2018) Cell cycle arrest through indirect transcriptional repression by p53: I have a DREAM. Cell Death Differ 25:114–132. https://doi.org/10.1038/cdd.2017.172
Liu X, Zhou Y, Liu X et al (2014) MPHOSPH1: a potential therapeutic target for hepatocellular carcinoma. Cancer Res 74:6623–6634. https://doi.org/10.1158/0008-5472.CAN-14-1279
Barsotti AM, Prives C (2009) Pro-proliferative FoxM1 is a target of p53-mediated repression. Oncogene 28:4295–4305. https://doi.org/10.1038/onc.2009.282
Gan L, Liu Z, Sun C (2018) Obesity linking to hepatocellular carcinoma: a global view. Biochim Biophys Acta Rev Cancer 1869:97–102. https://doi.org/10.1016/j.bbcan.2017.12.006
Elsegood CL, Tirnitz-Parker JE, Olynyk JK, Yeoh GC (2017) Immune checkpoint inhibition: prospects for prevention and therapy of hepatocellular carcinoma. Clin Transl Immunol 6:e161. https://doi.org/10.1038/cti.2017.47
Bachman KE, Herman JG, Corn PG et al (1999) Methylation-associated silencing of the tissue inhibitor of metalloproteinase-3 gene suggest a suppressor role in kidney, brain, and other human cancers. Cancer Res 59:798–802
Gill SE, Gharib SA, Bench EM et al (2013) Tissue inhibitor of metalloproteinases-3 moderates the proinflammatory status of macrophages. Am J Respir Cell Mol Biol 49:768–777. https://doi.org/10.1165/rcmb.2012-0377OC
Masson D, Rioux-Leclercq N, Fergelot P et al (2010) Loss of expression of TIMP3 in clear cell renal cell carcinoma. Eur J Cancer 46:1430–1437. https://doi.org/10.1016/j.ejca.2010.01.009
Nakamura M, Ishida E, Shimada K et al (2005) Frequent LOH on 22q12.3 and TIMP-3 inactivation occur in the progression to secondary glioblastomas. Lab Invest 85:165–175. https://doi.org/10.1038/labinvest.3700223
Pope C, Mishra S, Russell J, Zhou Q, Zhong XB (2017) Targeting H19, an imprinted long non-coding RNA, in hepatic functions and liver diseases. Diseases 5:E11. https://doi.org/10.3390/diseases5010011
Yoshimizu T, Miroglio A, Ripoche MA et al (2008) The H19 locus acts in vivo as a tumor suppressor. Proc Natl Acad Sci USA 105:12417–12422. https://doi.org/10.1073/pnas.0801540105
Li X, Wang H, Yao B, Xu W, Chen J, Zhou X (2016) lncRNA H19/miR-675 axis regulates cardiomyocyte apoptosis by targeting VDAC1 in diabetic cardiomyopathy. Sci Rep 6:36340. https://doi.org/10.1038/srep36340
Steck E, Boeuf S, Gabler J et al (2012) Regulation of H19 and its encoded microRNA-675 in osteoarthritis and under anabolic and catabolic in vitro conditions. J Mol Med 90:1185–1195. https://doi.org/10.1007/s00109-012-0895-y
Menghini R, Casagrande V, Menini S et al (2012) TIMP3 overexpression in macrophages protects from insulin resistance, adipose inflammation, and nonalcoholic fatty liver disease in mice. Diabetes 61:454–462. https://doi.org/10.2337/db11-0613
Vousden KH, Prives C (2009) Blinded by the light: the growing complexity of p53. Cell 137:413–431. https://doi.org/10.1016/j.cell.2009.04.037
Derdak Z, Villegas KA, Harb R, Wu AM, Sousa A, Wands JR (2013) Inhibition of p53 attenuates steatosis and liver injury in a mouse model of non-alcoholic fatty liver disease. J Hepatol 58:785–791. https://doi.org/10.1016/j.jhep.2012.11.042
Minamino T, Orimo M, Shimizu I et al (2009) A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat Med 15:1082–1087. https://doi.org/10.1038/nm.2014
Yokoyama M, Okada S, Nakagomi A et al (2014) Inhibition of endothelial p53 improves metabolic abnormalities related to dietary obesity. Cell Rep 7:1691–1703. https://doi.org/10.1016/j.celrep.2014.04.046
Taylor WR, Stark GR (2001) Regulation of the G2/M transition by p53. Oncogene 20:1803–1815. https://doi.org/10.1038/sj.onc.1204252
Laoukili J, Stahl M, Medema RH (2007) FoxM1: at the crossroads of ageing and cancer. Biochim Biophys Acta 1775:92–102. https://doi.org/10.1016/j.bbcan.2006.08.006
Pilarsky C, Wenzig M, Specht T, Saeger HD, Grutzmann R (2004) Identification and validation of commonly overexpressed genes in solid tumors by comparison of microarray data. Neoplasia 6:744–750. https://doi.org/10.1593/neo.04277
Alvarez-Fernandez M, Medema RH (2013) Novel functions of FoxM1: from molecular mechanisms to cancer therapy. Front Oncol 3:30. https://doi.org/10.3389/fonc.2013.00030
Wang SH, Ma F, Tang ZH et al (2016) Long non-coding RNA H19 regulates FOXM1 expression by competitively binding endogenous miR-342-3p in gallbladder cancer. J Exp Clin Cancer Res 35:160. https://doi.org/10.1186/s13046-016-0436-6
Lam EW, Brosens JJ, Gomes AR, Koo CY (2013) Forkhead box proteins: tuning forks for transcriptional harmony. Nat Rev Cancer 13:482–495. https://doi.org/10.1038/nrc3539
Pérez de Castro I, Aguirre-Portolés C, Fernández-Miranda G et al (2013) Requirements for Aurora-A in tissue regeneration and tumor development in adult mammals. Cancer Res 73:6804–6815. https://doi.org/10.1158/0008-5472.CAN-13-0586
Acknowledgements
This manuscript was in part funded by Associazione Italiana per la Ricerca sul Cancro (Grant AIRC IG-13163), MIUR PRIN 2015MPESJS_004 and Fondazione Roma NCD 2014 to M.F., Fondazione Roma NCD 2014 and EFSD/Boehringer Ingelheim 2017 to R.M. Horizon 2020 (Grant MADIA 732678), MoDiag Grant and Regione Lazio, Bando Life 2014–2020 to M.D. and I.A.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standard
The handling of mice and experimental procedures were conducted in accordance with experimental animal guidelines. Animal studies were approved by the University of Tor Vergata Animal Care and Use Committee.
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
For this type of study informed consent is not required.
Additional information
Managed by Antonio Secchi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Casagrande, V., Mauriello, A., Anemona, L. et al. Timp3 deficiency affects the progression of DEN-related hepatocellular carcinoma during diet-induced obesity in mice. Acta Diabetol 56, 1265–1274 (2019). https://doi.org/10.1007/s00592-019-01382-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-019-01382-x