Abstract
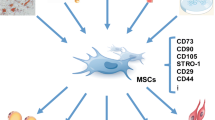
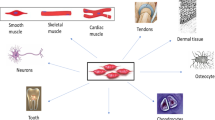
Diabetic nephropathy is the leading and possibly the most devastating complication of diabetes, with a prevalence ranging from 25 to 40 % in diabetic individuals, and as such represents an important challenge for public health worldwide. As a major cause of end-stage renal disease, diabetic nephropathy also accounts for a large proportion of deaths in diabetic individuals. To date, therapeutic options for overt diabetic nephropathy include medical interventions to reduce blood glucose levels and to control blood pressure and proteinuria. Recent evidence suggests a strong role for inflammation in the development and progression of diabetic nephropathy. Various immune cells, cytokines and chemokines have been implicated in the onset of diabetic nephropathy, while immune-related transcription factors and adhesion molecules have been correlated with the establishment of a renal proinflammatory microenvironment. Both inflammation and immune activation may promote severe distress in the kidney, with subsequent increased local fibrosis, ultimately leading to the development of end-stage renal disease. Stem cells are undifferentiated cells capable of regenerating virtually any organ or tissue and bearing important immunoregulatory and anti-inflammatory properties. Due to the aforementioned considerations, significant interest has been ignited with regard to the use of stem cells as novel therapeutics for diabetic nephropathy. Here, we will be examining in detail how anti-inflammatory properties of different populations of stem cells may offer novel therapy for the treatment of diabetic nephropathy.

Similar content being viewed by others
References
Williams WW et al (2012) Association testing of previously reported variants in a large case-control meta-analysis of diabetic nephropathy. Diabetes 61(8):2187–2194
Gross JL et al (2005) Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care 28(1):164–176
U.S. Renal Data System. USRDS 2010 Annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States. Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2010
Nathan DM et al (2005) Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 353(25):2643–2653
National Kidney F (2012) KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis 60(5):850–86
Astorri E et al (1997) Left ventricular function in insulin-dependent and in non-insulin-dependent diabetic patients: radionuclide assessment. Cardiology 88(2):152–155
Himmelfarb J, Tuttle KR (2013) New therapies for diabetic kidney disease. N Engl J Med 369(26):2549–2550
Paroni R et al (2005) Determination of asymmetric and symmetric dimethylarginines in plasma of hyperhomocysteinemic subjects. Amino Acids 28(4):389–394
Atkins RC, Zimmet P (2010) Diabetic kidney disease: act now or pay later. Acta Diabetol 47(1):1–4
Navarro-Gonzalez JF et al (2011) Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol 7(6):327–340
Macisaac RJ, Ekinci EI, Jerums G (2014) Markers of and risk factors for the development and progression of diabetic kidney disease. Am J Kidney Dis 63(2 Suppl 2):S39–S62
Galkina E, Ley K (2006) Leukocyte recruitment and vascular injury in diabetic nephropathy. J Am Soc Nephrol 17(2):368–377
Niewczas MA et al (2012) Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J Am Soc Nephrol 23(3):507–515
Mima A, Qi W, King GL (2012) Implications of treatment that target protective mechanisms against diabetic nephropathy. Semin Nephrol 32(5):471–478
Caramori ML et al (2002) Cellular basis of diabetic nephropathy: 1. Study design and renal structural-functional relationships in patients with long-standing type 1 diabetes. Diabetes 51(2):506–513
Fiorina P et al (2012) 31P-magnetic resonance spectroscopy (31P-MRS) detects early changes in kidney high-energy phosphate metabolism during a 6-month Valsartan treatment in diabetic and non-diabetic kidney-transplanted patients. Acta Diabetol 49(Suppl 1):S133–S139
Maffi P et al (2007) Kidney function after islet transplant alone in type 1 diabetes: impact of immunosuppressive therapy on progression of diabetic nephropathy. Diabetes Care 30(5):1150–1155
Bennett WM, Henrich WL, Stoff JS (1996) The renal effects of nonsteroidal anti-inflammatory drugs: summary and recommendations. Am J Kidney Dis 28(1 Suppl 1):S56–S62
Francese R, Fiorina P (2010) Immunological and regenerative properties of cord blood stem cells. Clin Immunol 136(3):309–322
Thomson JA et al (1998) Embryonic stem cell lines derived from human blastocysts. Science 282(5391):1145–1147
Fiorina P, Voltarelli J, Zavazava N (2011) Immunological applications of stem cells in type 1 diabetes. Endocr Rev 32(6):725–754
Abdi R et al (2008) Immunomodulation by mesenchymal stem cells: a potential therapeutic strategy for type 1 diabetes. Diabetes 57(7):1759–1767
Bussolati B et al (2005) Isolation of renal progenitor cells from adult human kidney. Am J Pathol 166(2):545–555
Rojas-Rios P, Gonzalez-Reyes A (2014) The plasticity of stem cell niches: A general property behind tissue homeostasis and repair. Stem Cells 32(4):852–859
D’Addio F, VVA, Ben Nasr M, Franek E, Zhu D, Li L, Ning G, Snarski E, Fiorina P (2014) Autologous non-myeloablative hematopoietic stem cell transplantation in new onset type 1 diabetes: a multicenter analysis. Diabetes (in press)
Doria A, Niewczas MA, Fiorina P (2012) Can existing drugs approved for other indications retard renal function decline in patients with type 1 diabetes and nephropathy? Semin Nephrol 32(5):437–444
Fernandez-Real JM et al (2012) Structural damage in diabetic nephropathy is associated with TNF-alpha system activity. Acta Diabetol 49(4):301–305
Lim AK, Tesch GH (2012) Inflammation in diabetic nephropathy. Mediators Inflamm 2012:146–154
Abbate M, Zoja C, Remuzzi G (2006) How does proteinuria cause progressive renal damage? J Am Soc Nephrol 17(11):2974–2984
RamachandraRao SP et al (2009) Pirfenidone is renoprotective in diabetic kidney disease. J Am Soc Nephrol 20(8):1765–1775
Remuzzi G, Benigni A, Remuzzi A (2006) Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J Clin Invest 116(2):288–296
Yu CC et al (2013) Abatacept in B7-1-positive proteinuric kidney disease. N Engl J Med 369(25):2416–2423
Fiorina P et al (2014) Role of Podocyte B7-1 in Diabetic Nephropathy. J Am Soc Nephrol [Epub ahead of print]
Sharma K et al (1996) Neutralization of TGF-beta by anti-TGF-beta antibody attenuates kidney hypertrophy and the enhanced extracellular matrix gene expression in STZ-induced diabetic mice. Diabetes 45(4):522–530
Lim AK et al (2009) Antibody blockade of c-fms suppresses the progression of inflammation and injury in early diabetic nephropathy in obese db/db mice. Diabetologia 52(8):1669–1679
Kanamori H et al (2007) Inhibition of MCP-1/CCR2 pathway ameliorates the development of diabetic nephropathy. Biochem Biophys Res Commun 360(4):772–777
Adhikary L et al (2004) Abnormal p38 mitogen-activated protein kinase signalling in human and experimental diabetic nephropathy. Diabetologia 47(7):1210–1222
Sanchez AP, Sharma K (2009) Transcription factors in the pathogenesis of diabetic nephropathy. Expert Rev Mol Med 11:e13
Wada J, Makino H (2013) Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci (Lond) 124(3):139–152
Okada S et al (2003) Intercellular adhesion molecule-1-deficient mice are resistant against renal injury after induction of diabetes. Diabetes 52(10):2586–2593
Lin M et al (2012) Toll-like receptor 4 promotes tubular inflammation in diabetic nephropathy. J Am Soc Nephrol 23(1):86–102
Drukker M et al (2002) Characterization of the expression of MHC proteins in human embryonic stem cells. Proc Natl Acad Sci USA 99(15):9864–9869
Drukker M et al (2006) Human embryonic stem cells and their differentiated derivatives are less susceptible to immune rejection than adult cells. Stem Cells 24(2):221–229
Bonde S, Zavazava N (2006) Immunogenicity and engraftment of mouse embryonic stem cells in allogeneic recipients. Stem Cells 24(10):2192–2201
Fandrich F et al (2002) Preimplantation-stage stem cells induce long-term allogeneic graft acceptance without supplementary host conditioning. Nat Med 8(2):171–178
Blum B, Benvenisty N (2009) The tumorigenicity of diploid and aneuploid human pluripotent stem cells. Cell Cycle 8(23):3822–3830
Little MH (2006) Regrow or repair: potential regenerative therapies for the kidney. J Am Soc Nephrol 17(9):2390–2401
Riolobos L et al (2013) HLA engineering of human pluripotent stem cells. Mol Ther 21(6):1232–1241
Cohen DE, Melton D (2011) Turning straw into gold: directing cell fate for regenerative medicine. Nat Rev Genet 12(4):243–252
Morigi M et al (2010) Life-sparing effect of human cord blood-mesenchymal stem cells in experimental acute kidney injury. Stem Cells 28(3):513–522
Chang JW et al (2011) Therapeutic effects of umbilical cord blood-derived mesenchymal stem cell transplantation in experimental lupus nephritis. Cell Transpl 20(2):245–257
Masoad RE et al (2012) Effect of mononuclear cells versus pioglitazone on streptozotocin-induced diabetic nephropathy in rats. Pharmacol Rep 64(5):1223–1233
Gammaitoni L et al (2004) Elevated telomerase activity and minimal telomere loss in cord blood long-term cultures with extensive stem cell replication. Blood 103(12):4440–4448
Romagnani P, Lasagni L, Remuzzi G (2013) Renal progenitors: an evolutionary conserved strategy for kidney regeneration. Nat Rev Nephrol 9(3):137–146
Lin F (2008) Renal repair: role of bone marrow stem cells. Pediatr Nephrol 23(6):851–861
Romagnani P, Remuzzi G (2013) Renal progenitors in non-diabetic and diabetic nephropathies. Trends Endocrinol Metab 24(1):13–20
Chhabra P, Brayman KL (2009) The use of stem cells in kidney disease. Curr Opin Organ Transpl 14(1):72–78
Ezquer FE et al (2008) Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biol Blood Marrow Transpl 14(6):631–640
Zhou H et al (2009) Mesenchymal stem cells transplantation mildly ameliorates experimental diabetic nephropathy in rats. Chin Med J (Engl) 122(21):2573–2579
Wang S et al (2013) Mesenchymal stem cells ameliorate podocyte injury and proteinuria in a type 1 diabetic nephropathy rat model. Biol Blood Marrow Transpl 19:538–546
Luz-Crawford P et al (2012) Mesenchymal stem cells repress Th17 molecular program through the PD-1 pathway. PLoS One 7(9):e45272
Spaggiari GM et al (2008) Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood 111(3):1327–1333
Krampera M et al (2003) Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 101(9):3722–3729
D’Addio F et al (2011) The link between the PDL1 costimulatory pathway and Th17 in fetomaternal tolerance. J Immunol 187(9):4530–4541
Guleria I et al (2007) Mechanisms of PDL1-mediated regulation of autoimmune diabetes. Clin Immunol 125(1):16–25
Aggarwal S, Pittenger MF (2005) Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105(4):1815–1822
Fang Y et al (2012) Autologous transplantation of adipose-derived mesenchymal stem cells ameliorates streptozotocin-induced diabetic nephropathy in rats by inhibiting oxidative stress, pro-inflammatory cytokines and the p38 MAPK signaling pathway. Int J Mol Med 30(1):85–92
Lee PY et al (2012) Induced pluripotent stem cells without c-Myc attenuate acute kidney injury via down-regulating the signaling of oxidative stress and inflammation in ischemia-reperfusion rats. Cell Transpl 21:2569–2585
Suarez-Alvarez B et al (2010) Epigenetic mechanisms regulate MHC and antigen processing molecules in human embryonic and induced pluripotent stem cells. PLoS One 5(4):e10192
Takahashi K et al (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131(5):861–872
Taylor CJ, Bolton EM, Bradley JA (2011) Immunological considerations for embryonic and induced pluripotent stem cell banking. Philos Trans R Soc Lond B Biol Sci 366(1575):2312–2322
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126(4):663–676
Pera MF (2011) Stem cells: the dark side of induced pluripotency. Nature 471(7336):46–47
Liang J et al (2010) Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: a pilot clinical study. Ann Rheum Dis 69(8):1423–1429
Acknowledgments
Paolo Fiorina is the recipient of a JDRF Career Development Award, an ASN Career Development Award and an ADA Mentor-based Fellowship grant. P.F. is also supported by a Translational Research Program (TRP) grant from Boston Children’s Hospital, a Harvard Stem Cell Institute grant (“Diabetes Program” DP-0123-12-00) and an Italian Ministry of Health grant (RF-2010-2303119, RF-2010-2314794 and RF-FSR-2008-1213704). Francesca D’Addio is the recipient of an Italian Scientists and Scholars of North America Foundation (ISSNAF)-Fondazione Marche Fellowship.
Conflict of interest
Paolo Fiorina discloses Sorgente S.r.l stock ownership and Otsuka grant funding. Francesca D’Addio, Alessio Trevisani, Moufida Ben Nasr, Roberto Bassi, Basset El Essawy, Reza Abdi, Antonio Secchi declare that they have no conflict of interest.
Statement of human and animal rights
This article does not contain any studies with human subjects performed by the any of the authors. Animal studies were conducted in accordance with institutional and National Institutes of Health guidelines and Institutional Animal Care and Use Committee (IACUC) approval.
Statement of informed consent
We would like to mention that there are no patients in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Managed by Massimo Porta.
Francesca D’Addio and Alessio Trevisani have contributed equally to this work.
Rights and permissions
About this article
Cite this article
D’Addio, F., Trevisani, A., Ben Nasr, M. et al. Harnessing the immunological properties of stem cells as a therapeutic option for diabetic nephropathy. Acta Diabetol 51, 897–904 (2014). https://doi.org/10.1007/s00592-014-0603-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-014-0603-1