Abstract
Purpose
Multiple different materials are used for filling bone defects following bone–patellar tendon–bone (BPTB) graft ACL reconstruction surgery. The theoretical objective being to minimize kneeling pain, improve clinical outcomes and reduce anterior knee pain following surgery. The impact of these materials is assessed in this study.
Methods
A prospective monocentric cohort study was conducted from January 2018 to March 2020. There were 128 skeletally mature athletic patients who underwent ACL reconstruction using the same arthroscopic-assisted BPTB technique, with a minimum follow-up of two years identified in our database. After obtaining approval from the local ethics committee, 102 patients were included in the study. Patients were divided into three groups based on type of bone substitute. The Bioactive glass 45S5 ceramic Glassbone™ (GB), collagen and hydroxyapatite bone void filler in sponge form Collapat® II (CP), and treated human bone graft Osteopure®(OP) bone substitutes were used according to availability. Clinical evaluation of patients at follow-up was performed using the WebSurvey software. A questionnaire completed in the 2nd post-operative year included three items: The ability to kneel, the presence of donor site pain, and the palpation of a defect. Another assessment tool included the IKDC subjective score and Lysholm score. These two tools were completed by patients preoperatively, and postoperatively on three occasions (6 months, 1 year, and 2 years).
Results
A total of 102 patients were included in this study. In terms of Kneeling pain, the percentage of GB and CP patients’ who kneel with ease were much higher than that of OP patients (77.78%, 76.5% vs 65.6%, respectively). All three groups experienced an important increase in IKDC and Lysholm scores. There was no difference in anterior knee pain between the groups.
Conclusion
The use of Glassbone® and Collapat II® bone substitutes reduced the incidence of kneeling pain compared to Osteopure®. There was no influence of the bone substitute type on the functional outcome of the knee or on the anterior knee pain at two years of follow.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anterior cruciate ligament (ACL) injuries are among the most common knee injuries, and ACL reconstruction (ACLR) is a widely performed operation [1, 2]. The bone–patellar tendon–bone (BPTB) and hamstring tendon autografts are two of the most commonly used autografts for ACLR [3, 4]. Furthermore, BPTB autograft has long been the gold standard for treatment, as its bone blocks at both ends of the graft provide high fixation strength [5, 6]. Nevertheless, 15–60% of patients may complain of long-term post-operative anterior knee pain during daily living or physical activities. Kneeling pain and donor site defects are also frequently observed [7,8,9,10,11,12].
It has been argued that patellar and tibial bone defects following graft harvesting are a risk factor impacting anterior knee pain in BPTB patients. Other claims are that infrapatellar nerve damage during graft harvesting is responsible for this morbidity [13, 14]. Recently, a systematic review showed that BTBP ACLR patients, whose bone defects were filled, have fewer post-operative knee complaints and better knee functional outcomes than patients treated without defect filling [8]. The most common bone grafts used are either autologous bone grafts, allogeneic bone grafts or synthetic substitutes [15,16,17]. Nonetheless, no study has compared the outcomes of different types of bone graft in terms of kneeling and functional outcomes in BTBP ACLR patients.
Such bone grafting options include the Bioactive glass 45S5 ceramic, such as Glassbone® (GB); collagen and hydroxyapatite bone void filler in sponge form, such as Collapat II® (CP), and treated human bone graft, such as Osteopure® (OP).
This cohort study aimed to investigate the influence of these bone graft types on kneeling and knee functional outcomes. The hypothesis was that there was no superiority of one substitute over another.
Materials and methods
A prospective single-center cohort study of the French prospective ACL Study [FAST] (NCT02511158) was performed, including all patients who performed ACLR using BPTB autograft between 2018 and 2020 by 4 senior surgeons. The study was approved by the local ethics committee (Comité de Protection des Personnes IDF VI), and informed consent was obtained from all patients. A retrospective analysis of the prospectively filled data, with a minimum follow-up of two years was performed. One hundred and two patients undergoing ACL reconstruction using BPTB autograft were assessed. Clinical evaluation of patients at follow-up was performed by the surgeons and data was entered in the WebSurvey software. The inclusion criteria were ACL reconstruction using the BTBP technique, athletes, a minimum of two years of post-operative follow-up and an age over 18 years. Exclusion criteria were associated knee ligament injury requiring surgical treatment, chondropathy of grade III or higher involving the trochlea or the patella, immune rheumatologic pathologies, preexisting anterior knee pain, and prior surgery on the same knee. Patients were divided into three groups according to bone substitute type. Three different bone substitutes were used according to availability at the time of surgery: Glassbone™, Collapat® II, and Osteopure®. The timeline is detailed in Fig. 1.
Bone substitutes
Osteopure® is a bone allograft harvested from a resected live human femoral head, and treated by sterilization at 25 kGy.
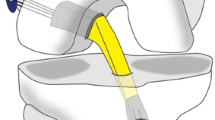
Glassbone® is a bioactive glass which is 100% synthetic, biocompatible, and osteoconductive and can integrate with the bone and soft tissue as a defect filler (Fig. 2). It is composed of a mixture of oxides (45% SiO2, 24.5% CaO, 25.5%, Na2O, and 6% P2O5 in weight %) [18,19,20,21,22,23,24,25].
Collapat® II is a bone void filler presented in spongy form. It is composed of a collagen structure in which hydroxyapatite granules are dispersed. The granules of hydroxyapatite give the material its osteoconductive properties [23].
Patient follow-up and data collection
Three tools were used for data collection at various time points. First, a questionnaire assessed the international knee documentation committee (IKDC) [24] subjective score and Lysholm score [25]. These two tools were completed by patients at four time points: first pre-operatively, and at 6 months, one year and two years postoperatively. Another standardized questionnaire was sent by email to the participants 4 months postoperatively. This was repeated at the 6 months, one year and two years marks following surgery. This questionnaire was made available online via a link to the WebSurvey software (websurvey.fr). If patients failed to answer, a first reminder was made via email, and a second by telephone call. Finally, a questionnaire was sent at the second post-operative year. It evaluated 3 items: The ability to kneel assessed by the subsection of Hacken’s questionnaire [26], the presence of donor site pain during sports or daily activities assessed by the Numerical Rating Scales (NRS) [27], and the sensation of a defect at the donor site. No formal sample size calculation was done. All eligible patients who underwent ACLR BPTB graft between 2018 and 2020 at our institution were included in the study.
Surgical protocol
Under spinal anesthesia, BPTB autografts were used to reconstruct the ACL. A 9 cm para median incision was performed, the paratenon was dissected carefully, and the a middle third of the patellar tendon was harvested with approximately 10 × 10 × 20 mm bone blocks from the patella and tibia. The ACL remnant was preserved. The tibial bone tunnel was prepared to be 10 mm in diameter. The tibial tunnel was created with a specific guide (Acufex; Smith & Nephew). The femoral tunnel was 10 mm in diameter and placed at the origin of the native ACL, on the medial surface of the lateral femoral condyle using an inside-out technique. The BTB autograft was fixed in the femoral tunnel with a non-absorbable interference screw (Softsilk; Smith & Nephew) or absorbable pins using the RigidFix system (DePuy Synthes, Mitek rigid fix), depending on surgeon preference. After tensioning the graft, the patellar bone block was stabilized in the tibial tunnel with another interference bioabsorbable screw (Smith & Nephew). Finally, the bony defects were filled with the corresponding bone graft and the paratenon was sutured over the bone substitutes.
Post-operative rehabilitation protocol
All patients underwent the same rehabilitation protocol. Immediate full weight-bearing with an articulated brace was allowed using crutches for the first 3 weeks to avoid unexpected falls. Physiotherapy for analgesia, patella mobilization, progressive full range-of-motion exercises, and isometric quadriceps contraction exercises were allowed, with the expectation that at one-month postoperatively, the patient would have a normal gait, full extension and 110° of flexion. In the case of meniscal suture, knee flexion while weight-bearing was limited to 120° for two months postoperatively.
Statistical analysis
All statistical analyses were performed using the IBM SPSS statistics software. Categorical variables were summarized as frequencies and percentages. Continuous variables were presented as means, standard deviations and ranges. One-way ANOVA was used to compare the mean IKDC and Lysholm scores, as well as the change in these scores between the three groups. Repeated measures ANOVA was used to compare the IKDC and Lysholm scores at different time points within each group. Pearson’s Chi-square test or Fisher exact test were used to assess the association of gender, ability to kneel, and internal pain between the three groups. All tests were two-sided and a p < 0.05 was considered statistically significant.
Results
One hundred and seventeen patients underwent ACLR using BPTB autograft. Of those, 102 (87.17%) were included in this study, and 15 (12.83%) were excluded. Among the 102 patients, 36 (35.29%) patients were placed in the Glassbone® group (group 1), 34 (33.33%) in the Collapat II® group (group 2), and the remaining 32 (31.37%) in the Osteopure® group (group 3). The three groups had no significant differences in terms of age (p = 0.127) and gender (p = 0.511). The mean age was 32.17 ± 8.20 years. Men represented 78.43% of the studied population. Detailed demographic characteristics are described in Table 1.
Among the 102 patients studied, 27 (26.47%) complained of Kneeling pain. There was a significant difference between the three groups (p = 0.045), the percentage of Glassbone and Collapat patients’ who kneel comfortably was significantly higher than that of osteobank patients (77.78%, 76,5% vs 65.6%, respectively). Moreover, the percentage of osteobank patients who were unable to kneel on hard surfaces was higher than that of Glassbone and Collapat patients (8% vs 2,78; 2.94%, respectively).
In the study population, 31 (30.39%) patients had anterior knee pain with an average of 3.77 ± 1.50 on the NRS scale. The percentage of patients experiencing anterior knee pain was 30.56% (mean 3.64 ± 1.03), 29.41% (mean 3.80 ± 1.69), and 31.25% (mean 3.90 ± 1.85) in groups 1, 2 and 3, respectively (p value 0.987).
The clinical characteristics are described in Table 2.
The IKDC score was significantly improved in the three groups compared to the pre-operative status (P < 0.01), as detailed in Table 3.
In group 1, the mean IKDC score increased from 56.67 ± 14.43 (range 26–84) pre-operatively to 69.22 ± 9.54 (range 36–86), 76.42 ± 9.26 (range 54–89) and 81.17 ± 10.61 (range 55–97) at 6 months, 1 year and 2 years postoperatively respectively, with a statistically significant mean change of 24.50 ± 15.64 (range (− 4)–60) (p < 0.001).
In group 2, the mean IKDC score increased from 60.35 ± 15.28 (range 32–90) at pre-operative to 66.65 ± 14.14 (range 20–83), 74.82 ± 16.58 (range 26–99) and 81.18 ± 15.97 (range 26–100) at 6 months, 1 year and 2 years post-operative respectively, with a statistically significant mean change of 20.52 ± 15.55 (range (− 8)–55) (p < 0.001).
In group 3, the mean IKDC score increased from 53.63 ± 18.38 (range 13–84) at pre-operative to 66.31 ± 16.15 (range 33–95), 74.16 ± 15.89 (range 39–98) and 77.69 ± 16.79 (range 40–98) at 6 months, 1 year and 2 years post-operative respectively, with a statistically significant mean change of 24.06 ± 19.94 (range (– 15.0)–73) (p < 0.001).
There was no statistically significant difference in the mean IKDC score between the three groups (p > 0.05).
The evolution of IKDC score by time in the three groups is shown in Fig. 3.
The Lysholm score was significantly improved in the three groups compared to the pre-operative status (p < 0.01) as detailed in Table 4.
In group 1, the mean Lysholm score increased from 67.53 ± 15.18 (range 28–95) at pre-operative to 81.33 ± 11.26 (range 44–95), 86.53 ± 10.24 (range 60–99) and 89.78 ± 9.90 (range 52–100) at 6 months, 1 year and 2 years post-operative respectively, with a statistically significant mean change of 22.25 ± 15.21 (range (− 6)–66) (p < 0.001).
In group 2, the mean Lysholm score increased from 67.88 ± 18.06 (range 15–95) at pre-operative to 81.41 ± 16.02 (range 22–99), 85.68 ± 13.43 (31–100) and 87.18 ± 13.78 (range 30–100) at 6 months, 1 year and 2 years post-operative respectively, with a statistically significant mean change of 19.29 ± 14.18 (range (− 1)–80.0) (p < 0.001).
In group 3, the mean Lysholm score increased from 60.84 ± 20.61 (range 2–99) at pre-operative to 76.09 ± 13.32 (range 49–100), 80.78 ± 11.82 (range 56–98) and 85.16 ± 12.37 (range 56–100) at 6 months, 1 year and 2 years post-operative respectively, with a statistically significant mean change of 24.31 ± 21.93 (range (− 11)–80) (p < 0.001).
Similarly, to the IKDC score, no statistically significant difference in the mean Lysholm score between the three groups was detected (p > 0.05).
The evolution of Lysholm score by time in the three groups is shown in Fig. 4.
All patients in the study, having subjectively assessed their knees, found no sensation of a bony defect at 2 years follow-up (Table 2). One incidence of a superficial abscess at the surgical site was observed in group 2. In this patient, the substitute was excized and an extra-articular debridement was needed to manage the complication.
Discussion
This study was designed to compare outcomes of ACL reconstruction with a BPTB autograft using one of three bone substitutes to fill the harvested zone. The primary finding was that the patients who received synthetic bone grafts, (Glassbone or Collapat II) had a higher percentage of painless kneeling compared to those who had Osteopure allograft filling. However, there was no significant difference between the three groups in terms of IKDC, Lysholm, and anterior knee pain.
Kneeling pain was evaluated using one item of Hacken’s questionnaire [26]. The higher incidence of kneeling pain of patients of group 3 compared to patients from other groups might be due to the persistent inflammatory response or suboptimal bone consolidation caused by the Osteopure allograft [28]. The incidence of painless kneeling in this study was 73.53% overall, with 77.78% of the Glassbone patients reporting no pain. After reviewing the literature, it was found that this was higher than the study by Taylor et al. (62%) [29] and lower than the study by Hacken et al. (90.4%) [26]. In both of those studies, cancellous autograft had been used for filling the bone defects. On the other hand Barrenius [30], Leitge [31] and Liden [32] who did not fill the bone defects registered a higher incidence of kneeling pain than the findings of the present study.
From a cosmetic standpoint, filling the defect with allograft improves appearance, a common patient concern, and abolishes the sensation of a bone gap or defect at the donor site. This allows avoidance of a further patient complaint during follow-up visits [33].
A major concern with using BPTB autograft for ACLR is donor site morbidity, specifically anterior knee pain [26]. Surgeons have attempted to change the harvesting technique in order to decrease this complication [12, 34], others have elected to change the graft type, like Schande et al. who used serum albumin-coated bone allograft [35]. Cervelline et al. filled the donor sites with PRP gel [36]. Nelson et al. also described a new technique for filling the defect [37]. Naresh et al. elected to fill the defects with ceramic bone graft but the results were non-satisfactory [38]. Our study aimed to identify the influence of different types of bone substitutes on anterior knee pain and found similar results in all three groups. The results are comparable to the findings of a systematic review by Lameire et al. who showed that filling defects decreased anterior knee pain, kneeling pain and extension loss [8].
No study evaluated and compared the functional outcome and donor site morbidity between Glassbone, Collapat II, or Osteopure bone substitutes in the BPTB ACLR population. Although there are numerous scoring tools to quantify ACLR patients’ results [39], IKDC and Lysholm scores were chosen for this study, as they are standard instruments for evaluating patients postoperatively and two of the most commonly reported functional outcome scores [8, 26, 40]. Both scores in the present study showed satisfactory recovery in all three groups without significant difference between groups. Subjective IKDC ranged from 77 to 81 after two years following ACLR, and the Lysholm score ranged from 85 to 90. Comparing our results to the systematic review by Lameire et al., it is observed that the IKDC scores are similar, but the Lysholm scores are inferior [8]. Overall, however, it was determined that the type of bone substitute did not affect the functional knee outcome.
There was no complication reported in terms of wound healing except for a patient from group 2 who exhibited an extrusion of part of the bone substitute and needed surgical intervention. This case might be a coincidence, and conclusions cannot be drawn based on a single exceptional case. It is important to mention that this is the first study that showed the tolerance of donor sites to Glassbone in BPTB ACLR patients. There were no complications detected which might be due to its bacteriostatic activity [22]. No patellar fracture occurred in any patient of the three groups. This is similar to the results of Alexander et al. [41].
This study shows that the kneeling pain was higher in Osteopure group. We can only speculate about this discrepancy. Osteopure is a natural bone block which needs to be cut into shape to fill a defect. As a rigid substitute, it is more difficult to fully fill the defect with it compared to the other softer substitutes (Glassbone and Collapat). Furthermore, it is composed of cancellous bone. Perhaps the replacement of cortical bone from the patella and tibia with spongy bone from the bone substitute affects rigidity and therefore leads to more pain in this patient population. Bone graft healing is a sequential process involving inflammation revascularization, osteogenesis remodeling, and incorporation into the host skeleton to form a mechanically efficient structure so this process might be different between the three allograft types. Further studies would be needed to possibly give a more accurate response in the future.
The strengths of this study were the high response rate, the 2-year follow-up period and the prospective administration of questionnaires.
There were, however, several limitations. First, this was not a randomized trial, and it was not a blinded study. Although patients may have been blinded, the surgeons would not have been. Furthermore, the bone substitute used was done so based on availability, rather than random assignment. Secondly, although the operations were all performed in the same institution, different surgeons participated in the study and performed surgery. Moreover, concomitant meniscus injury was not part of the exclusion criteria. This likely affects standardization of the procedure and may cause variability in patient outcomes.
Conclusion
This study finds that there is a reduced incidence of kneeling pain and discomfort when using bone substitutes such as Glassbone® and Collapat II® compared to allografts such as Osteopure® at a 2-year follow-up. However, the choice of bone-filling material influences neither functional knee outcomes, nor anterior knee pain at 2 years postoperatively.
References
Fox JA, Pierce M, Bojchuk J, Hayden J, Bush-Joseph CA, Bach BR (2004) Revision anterior cruciate ligament reconstruction with non irradiated fresh-frozen patellar tendon allograft. Arthrosc J Arthrosc Relat Surg 20(8):787–794. https://doi.org/10.1016/j.arthro.2004.07.019
Gianotti SM, Marshall SW, Hume PA, Bunt L (2009) Incidence of anterior cruciate ligament injury and other knee ligament injuries: a national population-based study. J Sci Med Sport 12(6):622–627. https://doi.org/10.1016/j.jsams.2008.07.005
Chee MYK et al (2017) Outcome of patellar tendon versus 4-strand hamstring tendon autografts for anterior cruciate ligament reconstruction: a systematic review and meta-analysis of prospective randomized trials. Arthrosc J Arthrosc Relat Surg 33(2):450–463. https://doi.org/10.1016/j.arthro.2016.09.020
Xie X, Liu X, Chen Z, Yu Y, Peng S, Li Q (2015) A meta-analysis of bone–patellar tendon–bone autograft versus four-strand hamstring tendon autograft for anterior cruciate ligament reconstruction. Knee 22(2):100–110. https://doi.org/10.1016/j.knee.2014.11.014
Erickson BJ et al (2014) Anterior cruciate ligament reconstruction practice patterns by NFL and NCAA football team physicians. Arthrosc J Arthrosc Relat Surg 30(6):731–738. https://doi.org/10.1016/j.arthro.2014.02.034
Papageorgiou CD, Ma CB, Abramowitch SD, Clineff TD, Woo SL-Y (2001) A multidisciplinary study of the healing of an intraarticular anterior cruciate ligament graft in a goat model. Am J Sports Med 29(5):620–626. https://doi.org/10.1177/03635465010290051501
Hardy A, Casabianca L, Andrieu K, Baverel L, Noailles T (2017) Complications following harvesting of patellar tendon or hamstring tendon grafts for anterior cruciate ligament reconstruction: Systematic review of literature. Orthop Traumatol Surg Res 103(8):S245–S248. https://doi.org/10.1016/j.otsr.2017.09.002
Lameire DL, Khalik HA, Zakharia A, Kay J, Almasri M (2021) Bone grafting the patellar defect after bone-patellar tendon–bone anterior cruciate ligament reconstruction decreases anterior knee morbidity: a systematic review. Arthrosc J Arthrosc Relat Surg 37(7):2361–2376. https://doi.org/10.1016/j.arthro.2021.03.031
Pinczewski LA, Lyman J, Salmon LJ, Russell VJ, Roe J, Linklater J (2007) A 10-year comparison of anterior cruciate ligament reconstructions with hamstring tendon and patellar tendon autograft. Am J Sports Med 35(4):564–574. https://doi.org/10.1177/0363546506296042
Plancher KD, Steadman JR, Briggs KK, Hutton KS (1998) Reconstruction of the anterior cruciate ligament in patients who are at least forty years old. J Bone Joint Surg Am Vol 80(2):184–197. https://doi.org/10.2106/00004623-199802000-00005
Shaieb MD, Kan DM, Chang SK, Marumoto JM, Richardson AB (2002) A prospective randomized comparison of patellar tendon versus semitendinosus and gracilis tendon autografts for anterior cruciate ligament reconstruction. Am J Sports Med 30(2):214–220. https://doi.org/10.1177/03635465020300021201
Tsuda E, Okamura Y, Ishibashi Y, Otsuka H, Toh S (2001) Techniques for reducing anterior knee symptoms after anterior cruciate ligament reconstruction using a bone-patellar tendon-bone autograft. Am J Sports Med 29(4):450–456. https://doi.org/10.1177/03635465010290041201
Gaudot F, Leymarie J-B, Drain O, Boisrenoult P, Charrois O, Beaufils P (2009) Double-incision mini-invasive technique for BTB Harvesting: its superiority in reducing anterior knee pain following ACL reconstruction. Orthop Traumatol Surg Res 95(1):28–35. https://doi.org/10.1016/j.otsr.2008.09.006
Kartus J, Stener S, Lindahl S, Engström B, Eriksson BI, Karlsson J (1997) Factors affecting donor-site morbidity after anterior cruciate ligament reconstruction using bone-patellar tendon-bone autografts. Knee Surg Sports Traumatol Arthrosc 5(4):222–228. https://doi.org/10.1007/s001670050054
Aglietti P, Giron F, Buzzi R, Biddau F, Sasso F (2004) Anterior cruciate ligament reconstruction: bone-patellar tendon-bone compared with double semitendinosus and gracilis tendon grafts a prospective randomized clinical trial. J Bone Joint Surg Am Vol 86(10):2143–55
Mohtadi N, Chan D, Barber R, Paolucci EO (2015) A randomized clinical trial comparing patellar tendon, hamstring tendon, and double-bundle ACL reconstructions. Clin J Sport Med 25(4):321–331. https://doi.org/10.1097/JSM.0000000000000165
Nicholas SJ, D’Amato MJ, Mullaney MJ, Tyler TF, Kolstad K, McHugh MP (2004) A prospectively randomized double-blind study on the effect of initial graft tension on knee stability after anterior cruciate ligament reconstruction. Am J Sports Med 32(8):1881–1886. https://doi.org/10.1177/0363546504265924
Fernandes HR, Gaddam A, Rebelo A, Brazete D, Stan GE, Ferreira JMF (2018) Bioactive glasses and glass-ceramics for healthcare applications in bone regeneration and tissue engineering. Materials. https://doi.org/10.3390/ma11122530
Hench LL (2006) The story of Bioglass. J Mater Sci Mater Med 17(11):967–78. https://doi.org/10.1007/s10856-006-0432-z
Paderni S, Terzi S, Amendola L (2009) Major bone defect treatment with an osteoconductive bone substitute. La Chirurgia degli organi di movimento 93(2):89–96. https://doi.org/10.1007/s12306-009-0028-0
Piitulainen JM, Posti JP, Aitasalo KMJ, Vuorinen V, Vallittu PK, Serlo W (2015) Paediatric cranial defect reconstruction using bioactive fibre-reinforced composite implant: early outcomes. Acta Neurochir 157(4):681–687. https://doi.org/10.1007/s00701-015-2363-2
Tamami NA, Bawazeer N, Fieux M, Zaouche S, Tringali S (2020) Tolerance and safety of 45S5 bioactive glass used in obliteration procedures during middle ear surgery: preliminary results. Am J Otolaryngol 41(6):102542. https://doi.org/10.1016/j.amjoto.2020.102542
Chajra H et al (2008) Collagen-based biomaterials and cartilage engineering. Application to osteochondral defects. Bio-Med Mater Eng 18(1 Suppl):S33-45
Irrgang JJ et al (2001) Development and validation of the international knee documentation committee subjective knee form. Am J Sports Med 29(5):600–613. https://doi.org/10.1177/03635465010290051301
Lysholm J, Gillquist J (1982) Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med 10(3):150–154. https://doi.org/10.1177/036354658201000306
Hacken BA et al (2020) A novel scoring instrument to assess donor site morbidity after anterior cruciate ligament reconstruction with a patellar tendon autograft at 2-year follow-up using contemporary graft-harvesting techniques. Orthop J Sports Med 8(6):2325967120925482. https://doi.org/10.1177/2325967120925482
Hjermstad MJ et al (2011) Studies comparing numerical rating scales, verbal rating scales, and visual analogue scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage 41(6):1073–1093. https://doi.org/10.1016/j.jpainsymman.2010.08.016
Thomas MV, Puleo DA (2011) Infection, inflammation, and bone regeneration a paradoxical relationship. J D Res 90(9):9. https://doi.org/10.1177/0022034510393967
Taylor DC et al (2009) Patellar tendon versus hamstring tendon autografts for anterior cruciate ligament reconstruction. Am J Sports Med 37(10):1946–1957. https://doi.org/10.1177/0363546509339577
Barenius B, Nordlander M, Ponzer S, Tidermark J, Eriksson K (2010) Quality of life and clinical outcome after anterior cruciate ligament reconstruction using patellar tendon graft or quadrupled semitendinosus graft: an 8-year follow-up of a randomized controlled trial. Am J Sports Med 38(8):1533–1541. https://doi.org/10.1177/0363546510369549
Leitgeb J et al (2014) Primary anterior cruciate ligament reconstruction in athletes: a 5-year follow up comparing patellar tendon versus hamstring tendon autograft. Wien Klin Wochenschr 126(13–14):397–402. https://doi.org/10.1007/s00508-014-0550-4
Lidén M, Ejerhed L, Sernert N, Laxdal G, Kartus J (2007) Patellar tendon or semitendinosus tendon autografts for anterior cruciate ligament reconstruction: a prospective, randomized study with a 7-Year follow-up. Am J Sports Med 35(5):740–748. https://doi.org/10.1177/0363546506298275
Boszotta H, Prünner K (2000) Refilling of removal defects: impact on extensor mechanism complaints after use of a bone-tendon-bone graft for anterior cruciate ligament reconstruction. Arthrosc J Arthrosc Relat Surg 16(2):160–164. https://doi.org/10.1016/S0749-8063(00)90030-6
Koh E, Oe K, Takemura S, Iida H (2015) Anterior cruciate ligament reconstruction using a bone-patellar tendon–bone autograft to avoid harvest-site morbidity in knee arthroscopy. Arthrosc Tech 4(2):e179–e184. https://doi.org/10.1016/j.eats.2015.01.002
Schandl K et al (2016) Bone-albumin filling decreases donor site morbidity and enhances bone formation after anterior cruciate ligament reconstruction with bone-patellar tendon-bone autografts. Int Orthop 40(10):2097–2104. https://doi.org/10.1007/s00264-016-3246-8
Cervellin M, de Girolamo L, Bait C, Denti M, Volpi P (2012) Autologous platelet-rich plasma gel to reduce donor-site morbidity after patellar tendon graft harvesting for anterior cruciate ligament reconstruction: a randomized, controlled clinical study. Knee Surg Sports Traumatol Arthrosc 20(1):114–120. https://doi.org/10.1007/s00167-011-1570-5
Mead RN, Benedict R, Limpisvasti O (2019) A technique utilizing graftnet to fill graft donor sites in bone-patellar-bone anterior cruciate ligament reconstruction. Arthrosc Tech 8(12):e1469–e1472. https://doi.org/10.1016/j.eats.2019.07.029
Dhanakodi N, Thilak J, Varghese J, Menon KV, Varma H, Tripathy SK (2019) Ceramic bone graft substitutes do not reduce donor-site morbidity in ACL reconstruction surgeries: a pilot study. SICOT-J 5:14. https://doi.org/10.1051/sicotj/2019013
Johnson DS, Smith RB (2001) Outcome measurement in the ACL deficient knee–what’s the score? Knee 8(1):51–57. https://doi.org/10.1016/s0968-0160(01)00068-0
Ahmad SS et al (2017) Outcome measures in clinical ACL studies: an analysis of highly cited level I trials. Knee Surg Sports Traumatol Arthrosc 25(5):1517–1527. https://doi.org/10.1007/s00167-016-4334-4
Barié A, Sprinckstub T, Huber J, Jaber A (2020) Quadriceps tendon vs. patellar tendon autograft for ACL reconstruction using a hardware-free press-fit fixation technique: comparable stability, function and return-to-sport level but less donor site morbidity in athletes after 10 years. Arch Orthop Trauma Surg 140(10):1465–1474. https://doi.org/10.1007/s00402-020-03508-1
Funding
This study has no funding to declare.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
All authors have declared and signed that they have no conflict of interests.
Ethical approval
Animals are not involved.
Informed consent
Humans were involved, all procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. The Institutional review board CPP (comité pour la protection des personnes) CPP-Ile-de-France VI reviewed and approved the study protocol on 07/02/2013, see the certificate in the supplemental materials.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fares, A., Hardy, A., Bohu, Y. et al. The impact of bone graft type used to fill bone defects in patients undergoing ACL reconstruction with bone–patellar tendon–bone (BPTB) autograft on kneeling, anterior knee pain and knee functional outcomes. Eur J Orthop Surg Traumatol 34, 181–190 (2024). https://doi.org/10.1007/s00590-023-03624-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00590-023-03624-9