Abstract
Purpose
To describe the clinical outcomes beyond pain relief of high-frequency spinal cord stimulation at 10 kHz (10 kHz SCS) in a patient with cervical myelopathy and drug-resistant chronic neuropathic pain with spastic tetraparesis.
Methods
A patient with C3-C6 myelomalacia and spastic tetraparesis previously treated with decompressive laminectomy underwent implantation of 10 kHz SCS for pain management through a trial procedure followed by permanent implantation. Due to the presence of epidural fibrotic scar tissue in the area of the previous C3-C6 laminectomy, the leads could not be implanted at the cervical level; therefore, the leads were positioned at the thoracic level. Data were collected during routine follow-up visits up to 15 months after implantation.
Results
Since the trialing phase and during all follow-up visits, along with complete pain relief in the lower limbs, a recovery from spasms was observed with an improvement in motor function. The patient recovered from a sensation of stiffness and difficulty in movement, with a significant decrease in muscle tone, regaining confidence in walking, and no longer needing assistance even for long walking distances. Although all disabling and painful symptomatology in the upper limbs instead did not ameliorate, the Oswestry Disability Index (ODI) score decreased from 50% at baseline to 6%.
Conclusion
To our knowledge, recovery from spasms and motor improvement in a spastic tetraparesis patient has never been reported before with 10 kHz SCS and possibly this new stimulation paradigm may overcome some performance limitations of traditional low-frequency SCS (LF-SCS). Treatment eliminated spasms at the lower limbs but not at the upper ones, thus suggesting that the location of the epidural leads could affect outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cervical myelopathy (CM) is a common, debilitating spinal cord disorder, caused by compression of the spinal cord in the neck and characterized by an insidious onset with slow clinical deterioration with periods of relative stability or quiescence punctuated by a stepwise decline in neurologic function [1]. Prolonged compression of the spinal cord may cause ischemia at the compressed level; myelomalacia clinical presentation is variable and ranges from mild to severe symptoms, sometimes catastrophic and commonly include pain and stiffness, spasticity, gait disturbances, impaired dexterity, difficulty with fine motor tasks, upper and lower extremity dysesthesias and paresthesia. We describe here a case of a cervical myelomalacia secondary to chronic spinal cord compression with severe neuropathic pain and spastic tetraparesis accompanied by a complete inability to walk autonomously, who underwent implantation with high-frequency spinal cord stimulation at 10 kHz (10 kHz SCS). 10 kHz SCS therapy is paresthesia-free neurostimulation which provides pain relief without exacerbating the underlying paresthesia [2]: this paresthesia-independent paradigm of stimulation has already demonstrated safety and effectiveness for the treatment of back and leg pain [3], as well as efficacy in other neuropathic pain syndromes such as upper limb and neck pain [4, 5]. In our case, along with expected pain relief, the patient reported also significant motor improvement and spasms recovery.
Case study presentation
This 66-year-old male presented with C3-C6 myelomalacia and spastic tetraparesis previously treated with decompressive laminectomy two years before. The onset of symptoms dated one year earlier with motor impairment and sensory deficit with caudal distribution and subsequent involvement of the upper limbs. He reported burning and crampy pain in the upper and lower limbs with tingling and pinpricking sensation in the extremities, tactile allodynia and paresthesia. Painful symptomatology was accompanied by continuous debilitating spasticity. He described a continuous sensation of stiffness, spasms, and involuntary contractions with difficulty in movement and either he was able to walk short distances with the aid of a walker or forced on a wheelchair. Neurological examination revealed modest motor autonomy on the bed, increased muscle tone, and need for assistance in all postural steps. Good trunk control in a sitting position without support. Standing and walking were possible with double support for very short stretches with a sharp gait and wide base, Mingazzini position with all 4 limbs without however being able to maintain it with a prevalence of deficit on the left.
High-dose steroid treatment did not modify the neurological deficits and induced iatrogenic diabetes. Electromyography (EMG) and Motor Evoked Potentials (MEPs) showed suffering of the central motor pathway on both upper and lower limbs. Painful symptomatology was refractory to neuropathic pain medications (pregabalin 150 mgx2/die and amitriptyline 32 mg/die) while buprenorphine was suspended due to allergic reactions. To manage pain, we decided to treat the patient with 10 kHz SCS.
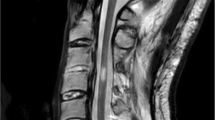
An initial assessment was performed to ensure that there were no clinical exclusion criteria for the SCS implant procedure, and MRI exam confirmed outcomes of C3-C6 laminectomy, and the cervical spinal cord showed extensive intramedullary hyperintense T2 signal at that level (Fig. 1). The leads could not be implanted in the cervical epidural space due to presence of fibrotic scar tissue in the area of previous laminectomy. Therefore, decision was made to implant leads epidurally only along T8 to T11 (midT9-top T8) (Fig. 2). The patient underwent temporary 10 kHz SCS trial stimulation for 3 weeks with percutaneous leads; after reporting pain relief at lower limbs > 50% using the Visual Analogue Scale (VAS), he underwent permanent SCS device implant (Nevro Senza® Omnia™, Nevro Corp., Redwood City, CA, USA), which included the 2 percutaneous leads placed epidurally connected to an implantable pulse generator (IPG) in the lower back. Stimulation parameters included 10-kHz frequency, 30-µs pulse width delivered via bipole, and amplitude range of 0.5 to 3.5 mA. Optimal bipole location and amplitude were adjusted per patient feedback, as previously described [3,4,5].
The immediate postoperative period was favorable and since trialing phase up to the last follow-up at 15 months the patient referred complete pain relief on the Visual Analogue Scale (VAS) at lower limbs, while upper limbs’ pain was stable; accordingly the Douleur Neuropathique en 4 Questionnaire (DN4) score changed from 7 at baseline (a score consistent with clinically confirmed neuropathic etiology of pain) to 0 at follow-up visits (Fig. 3). At admission the subject was in a severe disability category with a Oswestry Disability Index (ODI) score of 50% and a drastic improvement was observed in the disability level as the ODI score reduced to 6% since the first follow-up visit and remained stable up to the last follow-up at 15 months (Fig. 4). Along with a complete pain relief the patient experienced also a complete spasms relief at lower limbs, recovering from sensation of stiffness together with a significant functional improvement mostly related to walking ability, no longer needing assistance even for long-walking distances, therefore regaining confidence in walking. The level of muscle tone in each muscle group was assessed by Modified Ashworth Scale (MAS) scores in 3 joints of the patient’s lower and upper extremities with a 6-point (from 0 “No increase in muscle tone”, 1, 1+, 2, 3, to 4 “Affected parts rigid in flexion or extension”) scale. As shown in Fig. 5, the MAS scores of both legs gradually decreased, while no significant change was appreciated in the muscle tone of the upper extremities. Additionally, the change in painful symptoms compared with the baseline was assessed using the patient’s global impression of change (PGIC) Likert scale: relevant improvement of pain was achieved (“great and decisive improvement that makes the difference”) and improvement in the quality of sleep was reported. No perioperative complications or motor and sensory side effects during the follow up period were reported.
Indeed, all disabling and painful symptomatology in the upper limbs did not ameliorate and still represent a limitation.
Discussion
SCS therapy is a common treatment option for post laminectomy syndrome and complex regional pain syndrome [6]. With traditional low-frequency SCS (LF-SCS) therapeutic paresthesia is elicited in the painful dermatomes by applying electrical pulses to the spinal cord at frequencies ranging from 40 to 60 Hz. Successful masking of pain relies on concordance of the induced paresthesia with the painful area. However, the continuous perception of paresthesia during LF-SCS can be uncomfortable [7]. 10 kHz SCS is a paresthesia-free therapy whose mechanism of action is independent of paresthesia, thus providing pain relief at subsensory levels, which may be a more comfortable and desirable option for patients. Therefore as the described subject was suffering from upper and lower extremity dysesthesias and paresthesia, he was a good candidate for 10 kHz paresthesia-free stimulation.
This case of cervical myelomalacia secondary to prolonged spinal cord compression shows, along with complete pain relief, with 10 kHz SCS also an effect of SCS on spasticity. The stimulation aborted spasms rapidly and durably and the patient regained the ability to walk independently. The literature is replete mostly with various case reports all related to LF-SCS in over 25 different neurodegenerative, traumatic and other medical indications showing conflicting results on SCS providing relief of spasticity, suggesting that nature of the patient’s spasticity, frequency of the electrical stimulus and location of leads may be crucial for observing benefits on spasticity [8]. While 10 kHz SCS has demonstrated safety and effectiveness for the treatment of certain types of chronic pain [9, 10] and improved health-related quality of life [11], no published studies are testing it for motor spasticity. Indeed suggested mechanisms of action formulated for 10 kHz SCS for the primary outcomes (i.e. pain relief) include wide dynamic range neuron modulation, dorsal horn fiber recruitment, and local depolarization blockade [12, 13]. Some studies have suggested that 10 kHz SCS decreases wind-up and hyperpolarizes superficial dorsal horn neurons, suggesting segmental mechanisms that diverge from gate control theory. Another potential mechanism includes more profound activation of the interneuron pool without activating excitatory ones: this effect increases the inhibition of second-order neurons. This alternative mechanism of 10 kHz SCS compared to LF-SCS might play a role in spasticity modulation as this new paradigm of stimulation actually could bypass the performance-limitation of LF-SCS due to the layer of highly conductive cerebrospinal fluid (CSF) surrounding the spinal cord within the dura that constitutes an electrical current shunt that diminishes the epidural electrode field strengths. Therefore using LF-SCS the targeted structures positioned deeper within the spinal cord may not be excited without co-stimulation of the dorsal rootlets and other non-targeted structures and only a thin rim of the outermost neural tissues can be depolarized at physiologically acceptable levels of stimulation voltage.10 kHz SCS could represent a promising paradigm of stimulation with enough specificity to control spasticity reliably.
Cervical myelomalacia is usually associated with poor outcome. In our case, the prolonged cervical spinal cord compression led to C3-C6 myelomalacia documented at MRI with an increased T2 intramedullary intensity. After SCS, Patient-reported Outcome Measures showed an immediate significant improvement; also, complete relief of spasms with amelioration of spasticity of lower limbs was reported. All disabling and painful symptoms in the lower limbs improved since the trialing phase and remained stable up to the last follow-up visit at 15 months post implantation. Antispastic medications were already discontinued when he was referred to our surgery unit due to their inefficacy on symptomatology and side effects; the reported outcomes appear to be directly related to the 10 kHz therapy as during all the follow-up period no adjuvant measures, as rehabilitation and physiotherapy sessions, were implemented.
Conclusion
10 kHz spinal cord stimulation may improve motor function and walking ability in patients with spastic tetraparesis. In this case, along with complete pain relief in lower limbs, significant improvement in motor function and spasticity with muscle tone decreased to an almost normal level was observed after SCS implantation. Further investigations with a large number of patients are required to evaluate the validity of this technique to improve motor function in patients with spastic tetraparesis.
References
Lebl DR, Hughes A, Cammisa FP, O’Leary PF (2011) Cervical spondylotic myelopathy: pathophysiology, clinical presentation, and treatment. HSS J 7:170–178. https://doi.org/10.1007/s11420-011-9208-1
De Carolis G, Paroli M, Tollapi L, Doust M, Burgher AH, Yu C et al (2017) Paresthesia-Independence: an Assessment of technical factors related to 10 kHz paresthesia-free spinal cord stimulation. Pain Physician 20:331–341
Kapural L, Yu C, Doust MW, Gliner BE, Vallejo R, Sitzman BT et al (2015) Novel 10-kHz high-frequency therapy (HF10 therapy) is Superior to traditional low-frequency spinal cord stimulation for the treatment of Chronic Back and Leg Pain: the SENZA-RCT Randomized Controlled Trial. Anesthesiology 123:851–860. https://doi.org/10.1097/ALN.0000000000000774
Kapural L, Yu C, Doust MW, Gliner BE, Vallejo R, Sitzman BT et al (2016) Comparison of 10-kHz high-frequency and traditional low-frequency spinal cord stimulation for the treatment of Chronic Back and Leg Pain: 24-Month results from a Multicenter, Randomized, Controlled Pivotal Trial. Neurosurgery 79:667–677. https://doi.org/10.1227/NEU.0000000000001418
Amirdelfan K, Vallejo R, Benyamin R, Yu C, Yang T, Bundschu R et al (2020) High-frequency spinal cord stimulation at 10 kHz for the treatment of Combined Neck and Arm Pain: results from a prospective Multicenter Study. Neurosurgery 87:176–185. https://doi.org/10.1093/neuros/nyz495
Deer TR, Grider JS, Lamer TJ, Pope JE, Falowski S, Hunter CW et al (2020) A systematic literature review of spine neurostimulation therapies for the treatment of Pain. Pain Med 21:1421–1432. https://doi.org/10.1093/pm/pnz353
Hayek SM, Veizi E, Hanes M (2015) Treatment-limiting complications of Percutaneous spinal cord stimulator implants: a review of eight years of experience from an academic Center Database. Neuromodulation 18:603–608. https://doi.org/10.1111/ner.12312
Nagel SJ, Wilson S, Johnson MD, Machado A, Frizon L, Chardon MK et al (2017) Spinal cord stimulation for spasticity: historical approaches, current status, and future directions. Neuromodulation: Technol Neural Interface 20:307–321. https://doi.org/10.1111/ner.12591
Al-Kaisy A, Van Buyten JP, Smet I, Palmisani S, Pang D, Smith T (2014) Sustained effectiveness of 10 kHz high-frequency spinal cord stimulation for patients with chronic, low back Pain: 24-Month results of a prospective Multicenter Study. Pain Med 15:347–354. https://doi.org/10.1111/pme.12294
Stauss T, El Majdoub F, Sayed D, Surges G, Rosenberg WS, Kapural L et al (2019) A multicenter real-world review of 10 kHz SCS outcomes for treatment of chronic trunk and/or limb pain. Ann Clin Transl Neurol 6:496–507. https://doi.org/10.1002/acn3.720
Amirdelfan K, Yu C, Doust MW, Gliner BE, Morgan DM, Kapural L et al (2018) Long-term quality of life improvement for chronic intractable back and leg pain patients using spinal cord stimulation: 12-month results from the SENZA-RCT. Qual Life Res 27:2035–2044. https://doi.org/10.1007/s11136-018-1890-8
Sdrulla AD, Guan Y, Raja SN (2018) Spinal cord stimulation: clinical efficacy and potential mechanisms. Pain Pract 18:1048–1067. https://doi.org/10.1111/papr.12692
Chakravarthy K, Richter H, Christo PJ, Williams K, Guan Y (2018) Spinal cord stimulation for treating Chronic Pain: reviewing preclinical and clinical data on paresthesia-free high frequency therapy. Neuromodulation 21:10–18. https://doi.org/10.1111/ner.12721
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Conflict of interest
None of the authors has any potential conflict of interest.
Consent to participate
The procedures performed including 10 kHz SCS trial and implantation of the IPG was part of the usual treatment plan for patients with drug-resistant neuropathic pain and was not part of a prospective study requiring Institutional Review Board approval.
Consent to publish
The participant has consented to the submission of the case report to the journal.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gazzeri, R., Galarza, M. & Occhigrossi, F. Motor improvement and spasms recovery with high-frequency 10 kHz spinal cord stimulation in a patient with spastic tetraparesis: beyond pain relief. Eur Spine J (2024). https://doi.org/10.1007/s00586-024-08505-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00586-024-08505-1