Abstract
Purpose
Lymphocele formation following anterior lumbar interbody fusion (ALIF) is not common, but it can pose diagnostic and treatment challenges. The purpose of this case is to report for the first time the treatment of a postoperative lymphocele following a multi-level ALIF using a peritoneal window made through a minimally invasive laparoscopic approach.
Methods
Case report.
Results
A 74-year-old male with a history of prostatectomy and pelvic radiation underwent a staged L3–S1 ALIF (left paramedian approach) and T10-pelvis posterior instrumented with L1–5 decompression/posterior column osteotomies for degenerative scoliosis and neurogenic claudication. Three weeks after surgery, swelling of the left abdomen and entire left leg was reported. Computed tomography of the abdomen/pelvis demonstrated a large (19.2 × 12.0 × 15.4 cm) retroperitoneal fluid collection with compression of the left ureter and left common iliac vein. Fluid analysis (80% lymphocytes) was consistent with a lymphocele. Percutaneous drainage for 4 days was ineffective at clearing the lymphocele. For more definitive management, the patient underwent an uncomplicated laparoscopic creation of a peritoneal window to allow passive drainage of lymphatic fluid into the abdomen. Three years after surgery, he had no back or leg pain, had achieved spinal union, and had no abdominal swelling or left leg swelling. Advanced imaging also confirmed resolution of the lymphocele.
Conclusions
In this case report, creation of a peritoneal window minimally invasively via a laparoscope allowing passive drainage of lymphatic fluid into the abdomen was safe and effective for management of an abdominal lymphocele following a multi-level ALIF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anterior lumbar interbody fusion (ALIF) is an efficacious surgical technique to treat lumbar degenerative pathology, correct spinal deformity, and achieve spinal fusion. While it is relatively safe, it is associated with important complications, including incisional hernia, retrograde ejaculation, vascular and ureteral injury, and lymphocele formation [1,2,3]. While up to 25% of lymphoceles are identified incidentally and are without associated symptoms, symptomatic lymphoceles can manifest through abdominal pain and/or distension, wound drainage, and lower extremity pain and swelling secondary to compression of intrapelvic visceral structures [4]. Commonly utilized management strategies have relatively high recurrence rates, and a more durable option is preferred. Creation of an opening in the peritoneum through which lymphatic fluid can drain is a well-described technique to treat lymphoceles following renal transplantation; however, its use to manage lymphocele formation following ALIF has yet to be described. In this case report, we present the treatment of a postoperative lymphocele following a multi-level ALIF using a peritoneal window through a laparoscopic approach.
Case presentation
Initial presentation
A 74-year-old male presented with difficulty standing upright and progressive and disabling back and leg pain. Past medical history was notable for prior L4–5 decompression and prostate cancer managed by radical prostatectomy and pelvic radiation. Radiographs demonstrated multi-level lumbar spondylosis, grade 1 L4–5 spondylolisthesis, degenerative scoliosis and coronal plane deformity, and loss of lumbar lordosis/flatback (Fig. 1). Lumbar spine magnetic resonance imaging (MRI) was notable for lateral recess, central, and foramina stenosis from L1 to S1.
A Presenting full-spine radiographs demonstrated multi-level lumbar spondylosis, grade 1 spondylolisthesis at L4–5, degenerative scoliosis and coronal plane deformity, as well as a loss of lumbar lordosis/flatback resulting in lumbopelvic mismatch and a global sagittal plane deformity. After undergoing a L3–S1 anterior lumbar interbody fusion and T10 to pelvis posterior instrumented fusion with L1–5 decompression and T9 and T10 cement augmentation, there was improvement in regional and global coronal and sagittal alignment that were maintained 3 years after surgery (B)
Index spinal operations
The patient underwent a L3–S1 ALIF without complication at the time of the operation followed two days later by a T10 to pelvis posterior instrumented fusion with L1–5 decompression/posterior column osteotomies. The ALIF was performed through a left paramedian approach. The vascular surgeon noted, “A fair amount of inflammation and lymphatic tissue, which was divided and ligated using clips and/or ties.” Postoperatively, he had an uneventful course with no report of abdominal swelling or atypical incisional pain or abdominal pain. He was discharged home on postoperative day 6.
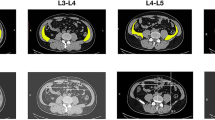
Three weeks post-op, he reported diffuse left leg swelling and a lump/“mass” of the left abdominal wall. Examination revealed swelling around the left paramedian incision that was slightly tender to touch, fluctuant, and fluid-filled. His entire left leg was also swollen with 3 + pitting edema. Abdominal pain, nausea, vomiting, wound drainage, fevers, and chills were not present. Abdomen/pelvis computed tomography (CT) demonstrated a large fluid collection in the retroperitoneal space measuring 19.2 cm × 12.0 cm × 15.4 cm (Fig. 2). There were also left hydronephrosis and left hydroureter with a change in the mid-abdomen secondary to compression from the fluid collection. Doppler ultrasound of the left lower extremity reported no deep vein thrombosis. Swelling of the left leg was presumed secondary to compression of the left common iliac vein.
Representative sagittal (A) and axial (B) computed tomography (CT) images of the abdomen/pelvis demonstrated a large fluid collection in the retroperitoneal space measuring 19.2 cm × 12.0 cm × 15.4 cm. Left hydronephrosis (red arrows) and left hydroureter (green arrow) secondary to compression of the left ureter by the fluid collection were seen (A, C). Compression of the left common iliac vein also manifested in diffuse swelling of the left leg
A percutaneous drain was placed in the fluid collection, which initially yielded 1100 cc of hazy yellow fluid. The fluid was sent for a variety of tests, the results of which are presented in Table 1. A lymphocytic predominance (~ 80%) was consistent with a lymphocele (Table 1). There was no evidence of infection (Table 1). Urinoma was ruled out given fluid creatinine levels lower than serum creatinine levels (Table 1). As drain output remained high (~ 1 L per day), an alternative treatment strategy, including repositioning of the drain, sclerotherapy, marsupialization, and creation of a peritoneal window via a laparoscopic approach were discussed. Given the latter’s efficacy in treating lymphoceles following renal transplants, it was pursued.
Laparoscopic peritoneal window (Fig. 3)
Intraoperative laparoscopic images showing the multi-loculated lymphocyte cavity with the previously placed drain within it (A; blue #). A large window (4 cm × 6 cm) was created in the peritoneum (green *) that allowed communication between the intraperitoneal cavity (red ^) and the lymphocele (blue #) (B, C)
The patient was brought to the operating room with one of our institution’s transplant surgeons. An infraumbilical incision was made first through which the peritoneal cavity was entered. Tacking sutures and a blunt Hasson trocar were placed, which was followed by insufflation. Two lateral 5-mm ports were placed on the right side of the abdomen to allow other instrumentation to be introduced contralateral to the fluid collection. A hook cautery and grasper were placed into the bulge and fluid was quickly seen to drain, consistent with entering into the lymphocele cavity. A wide window, approximately 4 cm × 6 cm, was made in the peritoneum. The largest opening was made without having to take down the descending colon. The previously placed drain was visualized sitting in the cavity as were multiple loculations, which were all broken up. The drain was removed. There was no obvious source of the lymphatic leak. Once good hemostasis and drainage were assured, the ports were removed and closed.
The following day, the patient reported improvement of abdominal and left leg swelling and was discharged home in stable condition.
Three-year follow-up
In follow-up, the patient’s function and activity level improved. At 3-year follow-up, he had no back and leg pain and was happy with his improved posture. Radiographs demonstrated intact instrumentation, union, and improved regional and global coronal and sagittal alignment (Fig. 1). There was also resolution of the left leg and abdominal swelling. Advanced imaging at 3 years after surgery demonstrated no recurrence of the fluid collection (Fig. 4).
Discussion
Anatomy of the lymphatic system is quite intricate. Lymph capillaries (which absorb lymph via intercellular gaps between endothelial cells of vascular walls) unite to form lymph vessels, which in turn join to form lymphatic trunks. There are five large lymphatic trunks on each side of the body, which coalesce to create two main lymphatic trunks. The largest of the two main lymphatic trunks is the thoracic duct (“ductus thoracicus”), which begins with the lumbar cistern and collects the fatty lymph from the abdominal organs (chyle). When lymphatic fluid collects in an anatomically unintended space (i.e., endothelial-free), it is termed a lymphocele. Lymphoceles often occur following surgical procedures in the pelvis and abdomen as a result of a lymphadenectomy, especially if the injured lymphatic vessels have not been closed completely. [5] The incidence of lymphocele in gynecological cancer surgeries has been reported to be greater than 20%, with 5.8% of patients experiencing symptoms related to the fluid collection [6]. Lymphoceles following ALIFs have been reported to occur in 2.1% of patients, with 1.6% reporting symptoms associated with the lymphocele [4]. This low rate of lymphocele formation in spine surgery may be attributed to collateral lymphatic pathways, rerouting lymphatic flow from the site of disruption, and reducing extravasated lymph [7]. However, injury to the lymphatic system may still result in extravasation of lymph from the iatrogenically severed lymphatic vessels surrounded by a thin pseudomembrane and the formation of a lymphocele, which is amber-colored sterile fluid [8]. If the lymphatic fluid drains through the skin via a dehiscent wound or the drainage site, a lymphatic fistula develops.
Risk factors for development of lymphoceles have reported to include older age, higher BMI, a greater number of levels fused, and ALIF levels at L2–L4 [4]. In addition to the number of levels accessed, risk factors for the development of our patient's lymphocele were prior pelvic surgery (radical prostatectomy) and radiation to the pelvis for prostate cancer. Note that our patient’s spine surgery was performed 7 months following his prostatectomy and 3 months following radiation to his pelvis. Differential diagnoses for lymphocele following an ALIF include infection, urinoma secondary to a ureteral injury, seroma, hematoma, cerebrospinal fluid leak (CSF), and lymphatic cysts. Lymphatic cysts usually start from a lymphangioma and occur as a result of secondary lymphedema. Rare internal lymphatic cysts are considered to be a congenital malformation of the large lymphatic vessels. When a lymphatic vessel is blocked, pressure builds up in it due to the pumping effect of the lymphangioma. In addition, lymphangiomas react to lymphatic congestion with increased contraction. Since the surrounding tissue and skin cannot withstand this pressure, the capillaries expand and cysts form [9]. Differentiation between these possibilities is accomplished with a CT scan, lymph or granulocyte scintigraphy, and analyzing the fluid for cell counts, gram stain, cultures, triglycerides, amylase, glucose, proteins, pH, creatinine, and beta-2 transferrin. A lymphocytic predominance in the fluid is consistent with a lymphocele, which was the case in our patients who had ~ 80% lymphocytes. The presence of a high neutrophil count and bacteria (positive culture and/or gram stain) would be consistent with an infection, while elevated creatinine in the fluid relative to the serum would be consistent with a urinoma. The presence of beta-2 transferrin in the fluid would suggest a CSF leak.
Most lymphoceles are asymptomatic and resolve spontaneously, as they usually regress on their own after a few weeks from neighboring lymph vessels transporting the lymph [10]. Symptoms associated with lymphoceles vary and depend on its size and location, but may include pelvic pain, leg pain, and/or leg swelling from peripheral lymphedema secondary to compromised lymphatic drainage, deep vein thrombosis, or compression of major intrapelvic veins. Symptomatic lymphoceles require intervention and can be treated with various methods, such as external drainage, aspiration, sclerotherapy, and surgical fenestration [11]. The current standard of care for symptomatic lymphoceles utilizes stepwise interventions, beginning with percutaneous drainage, laparoscopic marsupialization, and open surgical drain placement [12]. Sclerotherapy with ethanol, bleomycin, povidone-iodine, or tetracyclines, in conjunction with percutaneous drain placement, has also been described as a safe and cost-effective treatment with a 93–100% success rate [13, 14]. However, for lymphocytes that develop after renal transplantation, sclerotherapy with percutaneous drainage has demonstrated a recurrence rate of 20% [11, 15]. Surgical management remains reserved for cases refractory to percutaneous drain placement or minimally invasive procedures with persistently high output. Surgical marsupialization or fenestration can be performed via an open or laparoscopic approach. However, these approaches are more invasive and have associated complications of abdominal visceral injury and ureteral avulsion with a high recurrence rate [15].
In our case, we utilized a laparoscopic peritoneal window to manage a symptomatic lymphocele following a L3–S1 ALIF. Initial management was aimed at minimally invasive measures (i.e., percutaneous drainage); however, after several days of persistent high drainage from the drain, a more definitive surgical approach was felt to be indicated. As demonstrated, the laparoscopic peritoneal window in our patient was safe and minimally invasive, had minimal morbidity and limited associated hospital stay (1 day), and was ultimately highly effective, as there was complete resolution of the lymphocele. The "windowing" of the peritoneum, which strictly separates the abdominal cavity from the pelvic cavity, ensures that the accumulated lymphocele fluid drains from the pelvis into the abdomen. In the abdomen, the lymphatic fluid is then resorbed by the peritoneum, which acts as a form of a sponge. Lymphocele management in the setting of renal transplantation has been described using a laparoscopic peritoneal window to facilitate passive drainage of the lymphatic fluid leakage into the peritoneal cavity [16]. This operation is the most definitive method and is considered the treatment of choice for lymphocele management [16]. As such, creation of a peritoneal window should be a technique to which spine surgeons are privy so that it may advocated for if a lymphocele develops after an ALIF.
Conclusions
In this case report, creation of a peritoneal window minimally invasively via a laparoscope allowing passive drainage of lymphatic fluid into the abdomen was safe and effective for management of an abdominal lymphocele following a multi-level ALIF.
Data availability
All data generated or analyzed during this study are included in this published article.
Code availability
No software application or custom code was utilized.
References
Mobbs RJ, Phan K, Malham G, Seex K, Rao PJ (2015) Lumbar interbody fusion: techniques indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP. LLIF ALIF J Spine Surg 1(1):2–18
Phan K, Thayaparan GK, Mobbs RJ (2015) Anterior lumbar interbody fusion versus transforaminal lumbar interbody fusion: systematic review and meta-analysis. Br J Neurosurg 29(5):705–711
Hung NJ, Theologis AA, Courtier JL, Harmon D, Diab M (2021) Ureteral injury following anterior thoracolumbar spinal instrumented fusion for adolescent idiopathic scoliosis: a case report with CT angiography analysis of surgically relevant anatomy. Spine Deform 9(6):1691–1698
Scheer JK, Haddad AF, Chan AK et al (2021) Lymphocele after anterior lumbar interbody fusion: a review of 1322 patients. J Neurosurg Spine 35(6):722–728
Kay R, Fuchs E, Barry JM (1980) Management of postoperative pelvic lymphoceles. Urology 15(4):345–347
Zikan M, Fischerova D, Pinkavova I et al (2015) A prospective study examining the incidence of asymptomatic and symptomatic lymphoceles following lymphadenectomy in patients with gynecological cancer. Gynecol Oncol 137(2):291–298
Hanson D, Mirkovic S (1998) Lymphatic drainage after lumbar surgery. Spine 23(8):956–958
Levi AD (1999) Treatment of a retroperitoneal lymphocele after lumbar fusion surgery with intralesional povidone iodine: technical case report. Neurosurgery 45(3):658–660
Weinberger V, Cibula D, Zikan M (2014) Lymphocele: prevalence and management in gynecological malignancies. Expert Rev Anticancer Ther 14(3):307–317
Park SB, Kim JK, Cho KS (2007) Complications of renal transplantation: ultrasonographic evaluation. J Ultrasound Med 26(5):615–633
Lucewicz A, Wong G, Lam VWT et al (2011) Management of primary symptomatic lymphocele after kidney transplantation: a systematic review. Transplantation 92(6):663–673
Khorshidi F, Majdalany BS, Peters G et al (2021) Minimally invasive treatment of abdominal lymphocele: a review of contemporary options and how to approach them. Lymphology 54(2):56–67
Zuckerman DA, Yeager TD (1997) Percutaneous ethanol sclerotherapy of postoperative lymphoceles. AJR Am J Roentgenol 169(2):433–437
Alago W Jr, Deodhar A, Michell H et al (2013) Management of postoperative lymphoceles after lymphadenectomy: percutaneous catheter drainage with and without povidone-iodine sclerotherapy. Cardiovasc Intervent Radiol 36(2):466–471
Golriz M, Klauss M, Zeier M, Mehrabi A (2017) Prevention and management of lymphocele formation following kidney transplantation. Transplant Rev 31(2):100–105
Thurlow JP, Gelpi J, Schwaitzberg SD, Rohrer RJ (1996) Laparoscopic peritoneal fenestration and internal drainage of lymphoceles after renal transplantation. Surg Laparosc Endosc 6(4):290–295
Funding
No funding was acquired in support of this work.
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to the conception or design of the work, the acquisition, analysis, or interpretation of data, drafted the work or revised it critically for important intellectual content, approved the version to be published, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical approval
Ethics approval was waived by our institution’s Institutional Review Board.
Consent to participate
Consent to participate was obtained from the patient.
Consent for publication
Consent to publish was obtained from the patient.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Collins, A.P., Freise, C.E., Hiramoto, J. et al. Abdominal lymphocele following multi-level anterior lumbar interbody fusion (ALIF) managed with a laparoscopic peritoneal window: case report and review of the literature. Eur Spine J (2023). https://doi.org/10.1007/s00586-023-08072-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00586-023-08072-x