Abstract
Purpose
Although movement of the hyoid bone is different for masticatory swallowing and liquid swallowing in normal subjects, it has not been studied after cervical spine surgery. Therefore, we analyzed the swallowing dynamics of masticatory swallowing in anterior cervical spine disease surgery using foods that require chewing close to actual meals.
Methods
A day before and one week after the surgery, a videofluoroscopic swallow study (VFSS) was performed, and the distance of hyoid bone movement in the anterior and superior directions, amount of opening of the upper esophageal sphincter (UES), time of passage through the pharynx, number of swallows, and amount of pharyngeal residual were measured on the VFSS images during a masticatory swallow of corn flakes. The swallowing function was evaluated by DSS (dysphagia severity scale) and FOIS (functional oral intake scale). Imaging software was used for the measurements.
Results
Postoperative hyoid movement during masticatory swallowing was not significantly different for anterior movement but significantly limited in upward movement (p = 0.002); UES opening volume was significantly decreased (p < 0.001), and bolus residue was significantly worse (p < 0.001), compared to preoperative. The pharyngeal transit time was not significantly different; the number of swallows increased (p < 0.001), along with DSS (p < 0.001) and FOIS (p < 0.001), with significant differences before and after surgery, indicating worsened swallowing function.
Conclusions
Swallowing function worsened in masticatory swallowing after surgery for cervical spine disease, mainly due to the restriction of upward movement of the hyoid bone and the resulting increase in pharyngeal residuals after swallowing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anterior cervical spine surgery is a common procedure for most cervical spine lesions, including cervical disk herniation, cervical spondylotic myelopathy, and cervical ossification of the posterior longitudinal ligament. There are few serious complications that may occur after anterior cervical discectomy and fusion (ACDF). However, dysphagia is known as one of the common complications after ACDF. The incidence of dysphagia varies (2–83%) depending on the phase after surgery [1,2,3]. A previous systematic review reported that the incidence of dysphagia was higher in the acute phase and gradually decreased over time. The overall incidence of dysphagia within the first week after ACDF was averaged at 33.1%, and decreased to 19.8% 6 months after surgery [4]. Various causes for the development of postoperative dysphagia have been reported. These include edema of the esophageal or pharyngeal wall; postoperative retropharyngeal hematoma; displaced bone graft, cage, or plate; the use of recombinant human bone morphogenetic protein-2; and neurologic injury to the recurrent laryngeal nerve or superior laryngeal nerve [5]. However, prolonged dysphagia can be a significant risk factor for decreased quality of life in patients and poor nutrition. The incidence of chronic dysphagia has been reported to range from 3 to 35% [2].
In clinical practice, postoperative dysphagia can be a significant problem. Spine surgery in the cervical region may reduce the ability to elevate the larynx and contract the pharynx during swallowing because the operative field includes the supra- and infrahyoid muscle groups, which are involved in swallowing function. However, few clinical studies have focused on this perioperative swallowing function. Impaired swallowing increases the risk of postoperative aspiration pneumonia. Our previous study reported that 29.3% of patients developed dysphagia after anterior fusion surgery [3]. Postoperative complications can affect the length of hospital stay, rehabilitation period, and patient prognosis. Thus, perioperative assessment of swallowing function has become a major clinical topic related to cervical surgery. Swallowing indices such as the functional dysphagia scale, PAS (penetration aspiration scale) [6], pharyngeal retentions, and hyoid bone movement worsen after ACDF surgery [7]. The complications, including postoperative aspiration pneumonia and dysphagia due to relatively prolonged aspiration, are clinical problems that warrant management.
Since there are no studies reported on the detailed kinetics analysis of swallowing in patients with cervical spine diseases, we analyzed the swallowing dynamics during fluid intake with a videofluoroscopic swallow study (VFSS) in patients who had undergone anterior cervical fusion surgery and reported the factors associated with postoperative dysphagia. We found that an anterior-approached surgery resulted in significant limitation of hyoid bone movement, limited upper esophageal sphincter (UES) opening, and increased pharyngeal residual volume and number of swallows compared with preoperative surgery in liquid swallowing. On the other hand, the posterior-approached surgery results in limited forward movement of the hyoid bone compared with preoperative. We also reported that the postoperative procedure was associated with a significant increase in pharyngeal residual volume compared to the preoperative procedure, although it was less likely to cause restriction of swallowing dynamics [3]. Swallowing can be broadly divided into liquid and masticatory swallowing. There are distinct differences between the two, from feeding to the triggering of swallowing and airway defense. In liquid swallowing, the liquid is sent to the pharynx without going through food bolus formation after liquid ingestion. On the other hand, the tongue, soft palate, hyoid bone, and the mandible’s opening and closing mouth movements cooperate in chewing food and delivering it to the pharynx efficiently in masticatory swallowing. As for the hyoid bone, which is most involved in swallowing, previous studies in healthy subjects have reported that the amount of forward movement of the hyoid bone in masticatory swallowing is comparable to that in liquid swallowing, but the amount of upward movement is increased [8]. Therefore, it is not clear whether the large movement of the hyoid bone seen in masticatory swallowing, when impaired postoperatively, affects swallowing function or food intake. The study aimed to analyze the dynamics of masticatory swallowing using after anterior approach using solid foods, and to obtain knowledge on masticatory swallowing that is similar to actual eating situations.
Materials and methods
Participants
Between April 2019 and July 2022, the 50 study subjects who were considered for surgery (ACDF: anterior cervical discectomy and fusion, ACCF: anterior cervical corpectomy and fusion) in our orthopedic department consented to a before and after surgery VFSS of swallowing and were able to chew and swallow corn flakes (CF) were included. The subjects were patients with cervical degenerative diseases, such as cervical spondylotic myelopathy, cervical posterior longitudinal ligament ossification, cervical spondylotic amyotrophy, cervical disk herniation, and neoplastic lesions, who consulted the orthopedic surgery department of this university and for whom surgery was indicated. The number of fixed intervertebral segments was extracted from the subject’s medical record describing the surgical procedure. The exclusion criteria were as follows: (a) patients with a progressive disease that may cause dysphagia, (b) patients with structural or anatomical abnormalities due to previous surgery performed on the head and neck region, (c) patients with impaired consciousness and cognition or inability to follow instructions, and (d) patients whose consent for the study was not obtained. This study was approved by the Ethics Committee of the Dentistry Department of the Tokyo Medical and Dental University (approval number. D2019-004). The design and conduct of this study adhered to the general principles outlined in the Declaration of Helsinki. After providing sufficient written and verbal explanations, informed consent was obtained from all participants. The required sample size was set using "G*Power" with a significance level (α) of 0.05, power (1-β) of 0.8, and effect size (f) of 0.5, calculated as 28 participants required for the Wilcoxon test.
Data collection
The patients underwent VFSS the day before surgery and one week after surgery, and VFSS video data were analyzed when the subjects chewed and swallowed CF. VFSS in the lateral view was recorded with the subject in a sitting position using a digital X-ray TV system Flexavision (Shimadzu Medical Systems Corporation, Osaka, Japan). First, the participants were instructed to swallow 4.0 cc of moderately thick barium suspension (40% w/v) placed into the subject’s mouth with a syringe. Second, the participants chewed and swallowed the CF, which was made into about a spoonful of mixed and crushed 4 cc of thick barium water (40% w/v) of 2% added thickener [9].
Data analysis
The VFSS data were analyzed using Dipp-Motion V (ver1.2.2, DITECT Co., Tokyo, Japan) software. The analysis items were anterior and superior hyoid movement (mm), UES opening (mm), pharyngeal transit time (sec), the number of swallows, and pharyngeal residual volume (bolus residue scale: BRS; 6 levels [10]), which were evaluated using CF. Clinical severity classification of dysphagia (dysphagia severity scale; 7 levels [11,12,13]) and nutritional intake levels (functional oral intake scale; 7 levels [14]) were also evaluated with 4 cc of liquid and CF in the state of swallowing. Motion analysis of each video was performed by one dentist blinded to the data.
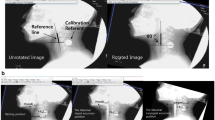
The position of the hyoid bone was P1 at the lowest point of the anterior surface of the second cervical vertebra (C2), P2 at the lowest point of the anterior surface of the fourth cervical vertebra (C4), and P3 at the most anterior point of the hyoid bone (Fig. 1A). The straight line connecting P1 and P2 is the vertical axis (Y axis), the straight line perpendicular to the Y axis and passing through P2 is the horizontal axis (X-axis). In the coordinate system with P2 as the origin, the most anterior point of the hyoid bone is X and Y expressed in coordinates (Fig. 1B). Then, point A was set to the position just before the sudden rise of the hyoid bone, point B was set to the top of the hyoid bone, point C was set to the anterior position of the hyoid bone, and point D was set to the resting point of the hyoid bone after swallowing. Using this XY plane, we plotted from the moment just before the rapid elevation of the hyoid bone to the resting position after swallowing. At the plotted points, forward and upward displacements (|Xc| − |Xa| and |Yb| − |Yd|) were measured (mm), respectively (Fig. 2). A 14.3-mm metal sphere was placed on the neck, and the X-ray image’s magnification was standardized each time [15].
The degree of UES opening was defined as the anterior–posterior length of the most constricted part of the esophagus between the height of the third (C3) and sixth (C6) cervical vertebrae. The VFSS image obtained when the extent of UES opening was the greatest during the barium swallow was used for measurement.
The pharyngeal transit time was adopted as the time required for the tip of the bolus to pass through the intersection of the root of the tongue and the inferior margin of the mandible and for the posterior margin of the bolus to pass through the esophageal entrance [16]. The number of swallows was defined as the number of swallows until the subject realized that the bolus was swallowed [17].
Statistical analysis
Statistical analysis was performed using SPSS Statistics (SPSS 25.0 J) from IBM (Chicago, IL, USA). Wilcoxon’s signed-rank test was used to determine whether there was a difference between the movements before and after surgery for each item. Participants were also classified into single-level and multilevel (≥ 2) fixed intervertebral counts based on previous studies [18], and the same items were compared pre- and postoperatively.
Results
Table 1 shows the participants’ characteristics. All 50 subjects were able to perform VFSS before and after surgery. The median age of the subjects was 59 years, with 37 males (74.0%) and 13 females (26.0%). Cervical disk herniation was the most common disease in 15 patients (30.0%). There was a tendency for postoperative anterior hyoid movement to be restricted compared to preoperative movement, but no significant difference was observed (p = 0.081). In contrast, upward movement was significantly limited (p = 0.002). The UES opening volume also significantly decreased (p < 0.001). Pharyngeal transit time increased compared to the preoperative period (p = 0.687), but the difference was insignificant. (Table 2; Fig. 3A–D).
Regarding the number of swallows, the percentage of those who swallowed a spoonful of CF ≥ 3 times postoperatively increased from 14% to 44.0%, significantly increasing postoperatively compared to preoperatively (p < 0.001). BRS was significantly increased (p < 0.001). Before and after surgery, BRS decreased from 96.0 to 50.0% for BRS1 (no residual after swallowing) and increased significantly from 2.0 to 20.0% for BRS4 (residual in the valleculae and posterior pharyngeal or piriform sinus after swallowing), respectively. There was a significant difference (p < 0.001) in DSS before and after surgery, indicating that aspiration was possible. The number of people aspirating (DSS ≤ 4) increased from 2 (4%) to 14 (28%) postoperatively. There was also a significant difference (p < 0.001) in FOIS before and after surgery. After surgery, FOIS 7 (normal diet level) decreased from 49 (98.0%) to 21 (42.0%). (Table 3; Fig. 4).
Divided by the number of fixed vertebrae, only upward hyoid shift, the number of swallows, and FOIS were significantly worse in patients at the single intervertebral level. The other items were not significantly different pre- and postoperatively. In contrast, in patients with multiple levels, swallowing dynamics and function worsened significantly postoperatively for all items (Table 4).
Discussion
Kinematic analysis of swallowing after surgery
During masticatory swallowing after cervical spine surgery, the anterior movement of the hyoid bone movement was not restricted, but the upward movement was significantly restricted. In general, the hyoid bone is raised anteriorly and upward by contraction of the suprahyoid muscle group, which activates the anti-aspiration mechanism by raising the food mass anteriorly and upward with the larynx before it reaches the hypopharynx [19]. In addition, the infrahyoid muscle group cooperates with the suprahyoid muscle group in hyoid bone movement.
In anterior cervical fusion, the supra- and infrahyoid muscles, such as the sternohyoid and scapulohyoid muscles, are indirectly invaded intraoperatively [20]. Therefore, the function of the suprahyoid and infrahyoid muscle groups that move the hyoid bone may be impaired within the first postoperative week due to edema of the perioperative tissues. Among the suprahyoid muscles, the geniohyoid muscles mainly move the hyoid bone anteriorly, and the digastric, mylohyoid, and stylohyoid muscles mainly move the hyoid bone upward. In the infrahyoid muscle group, the sternohyoid and scapulohyoid muscles are involved in the descent of the hyoid bone. Therefore, the infrahyoid muscle group may be more highly invaded than the suprahyoid muscle group, and upward and downward motion is considered more restricted than the anterior direction. The results of the present study suggest that it is important for spine and neurosurgeons to faithfully loosen retractors during anterior cervical operation to avoid excessive compression that can impair the function of these muscles and the inferior laryngeal nerve.
Postoperatively, the opening of the UES was significantly reduced. Three mechanisms are involved in the opening of the UES: relaxation of the cricopharyngeal muscle, contraction of the suprahyoid muscle group, elevation of the hyoid bone, and pressure of the food mass [21]. However, in the present study, no significant difference was observed in the forward movement of the hyoid muscles. Therefore, we considered that other factors influenced the UES opening. The contracting muscles of the hypopharynx are the thyropharyngeal and cricopharyngeal muscles innervated by the vagus nerve. The vagus nerve branches to the pharyngeal muscles run around the C3-4 [22] and may be invaded and disturbed if the upper cervical spine is included in the operative area. Another factor is the postoperative edema of the posterior wall of the pharynx [23]. It can be inferred that the postoperative thickening of the posterior pharyngeal wall physically compresses the upper esophageal sphincter. Also, intraoperative and postoperative compression of the pharyngeal plexus could be possible [24]. Increased edema of the posterior wall of the pharynx immediately after surgery decreases the contractility of the pharynx related to dysphagia [25]. Reports show that this pharyngeal edema is not always associated with dysphagia [26]. However, the study measured soft tissue thickness 2 weeks postoperatively, whereas we evaluated it 1 week postoperatively. Furthermore, the swallowing function may be more impaired immediately postoperatively and more susceptible to impaired passing of solid foods. Therefore, it was suggested that the significantly increased pharyngeal residue of solid foods postoperatively was caused by the impaired passage at the esophageal orifice due to the narrowing of the pharyngeal lumen caused by poor pharyngeal contraction and edema.
Our previous study showed that the number of fixed intervertebral spaces was related to the presence or absence of dysphagia in patients undergoing an anterior approach [3]. The greater the number of fixed multiple intervertebral segments, the more invasive is the surgical field, and the more likely it is that the hyoid motion and UES opening involved in swallowing will be restricted postoperatively. As a result, the PPT will likely be prolonged and pharyngeal residuals after masticatory swallowing will increase in multilevel compared to single-level.
Factors related to postoperative masticatory swallowing
In masticatory swallowing, before the swallowing reflex occurs, the masticated food is gradually delivered to the oropharynx (Stage II transport) before the start of the swallow reflex to form a food bolus [27]. The transport mechanism to the hypopharynx differs from that of liquid swallowing. In a study comparing the hyoid bone movement in masticatory and liquid swallowing, the amount of upward hyoid bone lifting during swallowing was reported to be significantly larger in masticatory swallowing than in liquid swallowing. This suggests that postoperative limitation of hyoid bone movement is more likely to affect masticatory swallowing. Based on these findings, patients with enough masticatory function to crush solid foods into a paste may be appropriate to start meals with solid foods that induce masticatory swallowing after anterior cervical spine surgery.
The number of swallows increased significantly postoperatively. It is considered that the food tended to remain in the pharynx because of the poor opening of the UES, and the swallowing frequency increased to remove the residue. In this study, the amount of cornflakes consumed was defined as one spoonful, or approximately 4 g. However, a larger amount could be ingested in actual meals or foods requiring more chewing than cornflakes. The risk of aspiration or choking due to increased pharyngeal residuals may increase in such cases. Particular attention should be paid to the ingestion of solid foods immediately after surgery.
Furthermore, pre-treatment rehabilitation for swallowing function after chemoradiotherapy for head and neck cancer was reported to significantly decrease the incidence of post-treatment dysphagia [28, 29]. In patients with cervical spine disease, preoperative rehabilitation might also decrease postoperative dysphagia. Training that is not stressful to the neck, such as tongue resistance training [30] and jaw-opening exercise [31], leading primarily to the training of the suprahyoid muscle group, may be effective.
Finally, the more invasive anterior approach procedures affect postoperative physical health [32,33,34]. Patients with preoperative dysphagia also experience prolonged and severe postoperative dysphagia [35]. Appropriate postoperative management, including assessment of swallowing function, helps stabilize the postoperative physical health and prevent aspiration. Furthermore, it enables the provision of a reasonable diet form for the patient, safe oral intake, and early discharge from the hospital [36, 37]. This study provides useful information that patients requiring multilevel surgery are more likely to have restricted hyoid motion and dysphagia than those with single-level surgery and are therefore more likely to require adjustments in diet form. Howells SR et al. stated that it was clinically important to manage patients’ swallowing function in collaboration with multiple professionals in the hospital during the acute phase and with speech therapists and dieticians in the community during the chronic phase [38]. It is required to manage postoperative dysphagia from the acute to the chronic postoperative period, and we believe that the results of this study, which analyzed the dynamics of chewing and swallowing solid foods similar to actual meals in detail, can provide useful information for orthopedic surgeons in their clinical practice.
Limitations
To our knowledge, this is the first study to investigate the kinematic details of masticatory swallowing dynamics after surgery for cervical spine disease. Several points were not fully investigated in this study. First, since we did not investigate the duration of mastication or the number of chews, whether postoperative mastication itself was affected was not determined. Therefore, future studies should also assess postoperative mastication. Second, the postoperative alignment of the neck changes, and the width of the pharyngeal cavity and the position of the hyoid bone may change anatomically as well, which could affect the swallowing function. Third, in the first postoperative week, peripharyngeal edema and tissue invasion around the cervical spine may not have fully recovered. Therefore, the swallowing function, including hyoid movement, is usually expected to improve over time. However, there are cases in which dysphagia persists for a long term, up to 12 weeks postoperatively. In such cases, the degree of dysphagia may also be severe. Future studies are needed to investigate the factors in patients with chronic dysphagia.
Conclusion
This study’s results indicate that the upward movement of the hyoid bone is restricted to masticatory swallowing postoperatively. In addition, the neuromuscular effects of surgery increase pharyngeal residuals postoperatively and worsen as swallowing dynamics. Despite the relatively minimally invasive nature of the surgery, nerves and muscles involved in swallowing run in or around the operative field, suggesting that physical pressure or other factors may have occurred.
References
Min Y, Kim W, Kang S, Choi J, Yeom J, Paik N (2016) Incidence of dysphagia and serial videofluoroscopic swallow study findings after anterior cervical discectomy and fusion: a prospective study. Clin Spine Surg 29:E177–E181. https://doi.org/10.1097/BSD.0000000000000060
Vaishnav AS, Saville P, McAnany S, Patel D, Haws B, Khechen B, Singh K, Gang CH, Qureshi SA (2019) Predictive factors of postoperative dysphagia in single-level anterior cervical discectomy and fusion. Spine (Phila Pa 1976) 44:E400–E407. https://doi.org/10.1097/BRS.0000000000002865
Yoshizawa A, Nakagawa K, Yoshimi K, Hashimoto M, Aritaki K, Ishii M, Yamaguchi K, Nakane A, Kawabata A, Hirai T, Yoshii T, Ikeda M, Okawa A, Tohara H (2022) Analysis of swallowing function after anterior/posterior surgery for cervical degenerative disorders and factors related to the occurrence of postoperative dysphagia. Spine J 23:513–522. https://doi.org/10.1016/j.spinee.2022.12.010
Riley LH 3rd, Vaccaro AR, Dettori JR, Hashimoto R (2010) Postoperative dysphagia in anterior cervical spine surgery. Spine (Phila Pa 1976) 35:S76–S85. https://doi.org/10.1097/BRS.0b013e3181d81a96
Joaquim AF, Murar J, Savage JW, Patel AA (2014) Dysphagia after anterior cervical spine surgery: a systematic review of potential preventative measures. Spine J 14:2246–2260. https://doi.org/10.1016/j.spinee.2014.03.030
Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL (1996) A penetration-aspiration scale. Dysphagia 11:93–98. https://doi.org/10.1007/BF00417897
Muss L, Wilmskoetter J, Richter K, Fix C, Stanschus S, Pitzen T, Drumm J, Molfenter S (2017) Changes in swallowing after anterior cervical discectomy and fusion with instrumentation: a presurgical versus postsurgical videofluoroscopic comparison. J Speech Lang Hear Res 60:785–793. https://doi.org/10.1044/2016_JSLHR-S-16-0091
Ishida R, Palmer JB, Hiiemae KM (2002) Hyoid motion during swallowing: factors affecting forward and upward displacement. Dysphagia 17:262–272. https://doi.org/10.1007/s00455-002-0064-5
Wakasugi Y, Tohara H, Hattori F, Motohashi Y, Nakane A, Goto S, Ouchi Y, Mikushi S, Takeuchi S, Uematsu H (2008) Screening test for silent aspiration at the bedside. Dysphagia 23:364–370. https://doi.org/10.1007/s00455-008-9150-7
Rommel N, Borgers C, Van Beckevoort D, Goeleven A, Dejaeger E, Omari TI (2015) Bolus residue scale: an easy-to-use and reliable videofluoroscopic analysis tool to score bolus residue in patients with dysphagia. Int J Otolaryngol 2015:780197. https://doi.org/10.1155/2015/780197
Tohara H, Palmer JB, Reynolds K, Kuhlemeier KV, Palmer S (2003) Dysphagia severity scale. Kokubyo Gakkai Zasshi 70:242–248. https://doi.org/10.5357/koubyou.70.242
O’Neil KH, Purdy M, Falk J, Gallo L (1999) The dysphagia outcome and severity scale. Dysphagia 14:139–145. https://doi.org/10.1007/PL00009595
Nishimura K, Kagaya H, Shibata S, Onogi K, Inamoto Y, Ota K, Miki T, Tamura S, Saitoh E (2015) Accuracy of Dysphagia Severity Scale rating without using videoendoscopic evaluation of swallowing. Jpn J Compr Rehab 6:124–128. https://doi.org/10.11336/jjcrs.6.124
Crary MA, Mann GD, Groher ME (2005) Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch Phys Med Rehab 86:1516–1520. https://doi.org/10.1016/j.apmr.2004.11.049
Hasegawa S, Nakagawa K, Yoshimi K, Yamaguchi K, Nakane A, Ishii M, Okumura T, Hara K, Minakuchi S, Tohara H (2022) Jaw-retraction exercise increases anterior hyoid excursion during swallowing in older adults with mild dysphagia. Gerodontology 39:98–105. https://doi.org/10.1111/ger.12595
Logemann JA, Shanahan T, Rademaker AW, Kahrilas PJ, Lazar R, Halper A (1993) Oropharyngeal swallowing after stroke in the left basal ganglion/internal capsule. Dysphagia 8:230–234. https://doi.org/10.1007/BF01354543
Nishi M, Takehara I, Ikai T, Miyano S (2006) Effects of nasogastric tubes on swallowing: frequency of swallowing, residue and back flow of bolus. Jpn J Rehab Med 43:243–248. https://doi.org/10.2490/jjrm1963.43.243
Oh LJ, Ong S, Ghozy S, Dmytriw AA, Zuccato J, Mobbs R, Phan K, Dibas M, Faulkner H (2020) Dysphagia rates in single- and multiple-level anterior cervical discectomy and fusion surgery: a meta-analysis. J Spine Surg 6:581–590. https://doi.org/10.21037/jss-20-506
Li Q, Minagi Y, Hori K, Kondoh J, Fujiwara S, Tamine K, Inoue M, Maeda Y, Chen Y, Ono T (2015) Coordination in oro-pharyngeal biomechanics during human swallowing. Physiol Behav 147:300–305. https://doi.org/10.1016/j.physbeh.2015.05.004
Ito S, Fukao S, Nonoyama Y, Fujita T, Kidooka M (2022) Dysphasia after anterior upper cervical discectomy and fusion due to paresis of the internal ramus of superior laryngeal nerve—a case report. Spinal Surgery 36:62–65. https://doi.org/10.2531/spinalsurg.36.62
Ono M, Tsukuda M, Kawai S, Mochimatsu I, Hirose H, Kagesato Y (2003) Two cases with impairment of opening of the upper esophageal sphincter (UES) by affection of the vagal nerve. Otol Fukuoka 49:459–463. https://doi.org/10.11334/jibi1954.49.6_459
Iwanaga J, Yilmaz E, Loukas M, Xu L, Uz A, Walocha JA, Dumont AS, Tubbs RS (2022) An anatomical study of the pharyngeal plexus: Application to avoiding postoperative dysphagia following anterior approaches to the cervical spine. Clin Anat 35:264–268. https://doi.org/10.1002/ca.23785
Kim SW, Jang C, Yang MH, Lee S, Yoo JH, Kwak YH, Hwang JH (2017) The natural course of prevertebral soft tissue swelling after anterior cervical spine surgery: how long will it last? Spine J 17:1297–1309. https://doi.org/10.1016/j.spinee.2017.05.003
Gutierrez S, Iwanaga J, Pekala P, Yilmaz E, Clifton WE, Dumont AS, Tubbs RS (2021) The pharyngeal plexus: an anatomical review for better understanding postoperative dysphagia. Neurosurg Rev 44:763–772. https://doi.org/10.1007/s10143-020-01303-5
Leonard R, Belafsky P (2011) Dysphagia following cervical spine surgery with anterior instrumentation: evidence from fluoroscopic swallow studies. Spine (Phila Pa 1976) 36:2217–2223. https://doi.org/10.1097/BRS.0b013e318205a1a7
Kepler CK, Rihn JA, Bennett JD, Anderson DG, Vaccaro AR, Albert TJ, Hilibrand AS (2012) Dysphagia and soft-tissue swelling after anterior cervical surgery: a radiographic analysis. Spine J 12:639–644. https://doi.org/10.1016/j.spinee.2012.03.024
Matsuo K, Saitoh E, Takeda S et al (2002) Effects of gravity and chewing on bolus position at swallow onset. Jpn J Dysphag Rehab 6:179–186. https://doi.org/10.32136/jsdr.6.2_179
Hsiang CC, Chen AW, Chen CH, Chen MK (2019) Early postoperative oral exercise improves swallowing function among patients with oral cavity cancer: a randomized controlled trial. Ear Nose Throat J 98:E73–E80. https://doi.org/10.1177/0145561319839822
Kato K (2020) Swallowing rehabilitation starting before surgery for patients with advanced oral cancer. Maxillofac Prosthet 43:14–18
Namiki C, Hara K, Tohara H, Kobayashi K, Chantaramanee A, Nakagawa K, Saitou T, Yamaguchi K, Yoshimi K, Nakane A, Minakuchi S (2019) Tongue-pressure resistance training improves tongue and suprahyoid muscle functions simultaneously. Clin Interv Aging 14:601–608. https://doi.org/10.2147/CIA.S194808
Wada S, Tohara H, Iida T, Inoue M, Sato M, Ueda K (2012) Jaw-opening exercise for insufficient opening of upper esophageal sphincter. Arch Phys Med Rehab 93:1995–1999. https://doi.org/10.1016/j.apmr.2012.04.025
Riley LH 3rd, Skolasky RL, Albert TJ, Vaccaro AR, Heller JG (2005) Dysphagia after anterior cervical decompression and fusion: prevalence and risk factors from a longitudinal cohort study. Spine (Phila Pa 1976) 30:2564–2569. https://doi.org/10.1097/01.brs.0000186317.86379.02
Lee MJ, Bazaz R, Furey CG, Yoo J (2007) Risk factors for dysphagia after anterior cervical spine surgery: a two-year prospective cohort study. Spine J 7:141–147. https://doi.org/10.1016/j.spinee.2006.02.024
Yoshii T, Morishita S, Inose H, Yuasa M, Hirai T, Okawa A, Fushimi K, Fujiwara T (2020) Comparison of perioperative complications in anterior decompression with fusion and posterior decompression with fusion for cervical ossification of the posterior longitudinal ligament: propensity score matching analysis using a nation-wide inpatient database. Spine (Phila Pa) 45:1006–1012. https://doi.org/10.1097/BRS.0000000000003469
Mazmudar A, Paziuk T, Tran KS, Henry T, Oh S, Purtill C, Habbal D, Yalla G, Harrill Q, Sherrod B, Bisson E, Brodke D, Kepler C, Schroeder G, Vaccaro A, Hilibrand A, Rihn JA (2023) Evaluating dysphagia duration and severity after ACDF in patients with underlying dysphagia—a prospective. Multicenter Study Glob Spine J 6:21925682231201250. https://doi.org/10.1177/21925682231201249
Carrión S, Roca M, Costa A, Arreola V, Ortega O, Palomera E, Serra-Prat M, Cabré M, Clavé P (2017) Nutritional status of older patients with oropharyngeal dysphagia in a chronic versus an acute clinical situation. Clin Nutr 36:1110–1116. https://doi.org/10.1016/j.clnu.2016.07.009
Gomes F, Emery PW, Weekes CE (2016) Risk of malnutrition is an independent predictor of mortality, length of hospital stay, and hospitalization costs in stroke patients. J Stroke Cerebrovasc Dis 25:799–806. https://doi.org/10.1016/j.jstrokecerebrovasdis.2015.12.017
Howells SR, Cornwell PL, Ward EC, Kuipers P (2019) Understanding dysphagia care in the community setting. Dysphagia 34:681–691. https://doi.org/10.1007/s00455-018-09971-8
Acknowledgements
The authors would like to thank the staff of the Department of Orthopaedics Surgery at Tokyo and Dental University Hospital for their cooperation in patient referrals and other activities.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aritaki, K., Nakagawa, K., Yoshimi, K. et al. Kinematic analysis of chewing and swallowing function after cervical spine surgery. Eur Spine J 33, 243–252 (2024). https://doi.org/10.1007/s00586-023-08022-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-023-08022-7