Abstract
Purpose
To review and describe the development, methods and cohort of the lumbosacral part of the Norwegian registry for spine surgery (NORspine).
Methods
NORspine was established in 2007. It is government funded, covers all providers and captures consecutive cases undergoing operations for degenerative disorders. Patients’ participation is voluntary and requires informed consent. A set of baseline-, process- and outcome-variables (3 and 12 months) recommended by the International Consortium for Health Outcome Measurement is reported by surgeons and patients. The main outcome is the Oswestry disability index (ODI) at 12 months.
Results
We show satisfactory data quality assessed by completeness, timeliness, accuracy, relevance and comparability. The coverage rate has been 100% since 2016 and the capture rate has increased to 74% in 2021. The cohort consists of 60,647 (47.6% women) cases with mean age 55.7 years, registered during the years 2007 through 2021. The proportions > 70 years and with an American Society of Anaesthesiologists’ Physical Classification System (ASA) score > II has increased gradually to 26.1% and 19.3%, respectively. Mean ODI at baseline was 43.0 (standard deviation 17.3). Most cases were operated with decompression for disc herniation (n = 26,557, 43.8%) or spinal stenosis (n = 26,545, 43.8%), and 7417 (12.2%) with additional or primary fusion. The response rate at 12 months follow-up was 71.6%.
Conclusion
NORspine is a well-designed population-based comprehensive national clinical quality registry. The register’s methods ensure appropriate data for quality surveillance and improvement, and research.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Norwegian Registry for Spine Surgery (NORspine) is a comprehensive national clinical quality registry established in 2007. Cases of patients operated for degenerative disorders in the lumbosacral and cervical spine are recorded in separate registers. This paper is the first of two and comprises the development and methods of the lumbosacral register. The subsequent paper discusses results of quality improvement and research based on data from the register.
Low back and neck pain are major causes of non-fatal health loss worldwide, and in Norway back pain is the most common cause for short-term sick leave and the second most common for disability benefits [1, 2]. A Cochrane review published in 1999 found conflicting evidence on the effectiveness of surgical treatment for degenerative lumbar disorders [3]. Still, 3460 to 4572 patients per year were operated in Norway for such disorders between 1999 and 2005 [4]. Insufficient quality assurance, lack of evidence-based clinical guidelines and the fact that spine surgery is preference-sensitive [5], has contributed to variation in surgical rates and techniques between hospitals, health regions and countries [6, 7].
Registries are useful for surveillance of quality, to attain more uniform and evidence-based clinical practices, as a data source for research, and for development of guidelines [8]. During the 1990s and 2000s, several spine registries emerged [8]. NORspine acquired inspiration from the Swedish spine registry (Swespine) which was the first in Scandinavia [9]. Some other are the Danish national lumbar spine database (DaneSpine), the European Spine Tango, the American Spine Registry, and the Australian Spine Registry [8, 10,11,12].
NORspine’s [13] objectives are to surveil and improve the quality of surgical treatment of degenerative conditions in the lumbosacral spine. Facilitation of and contribution to research are secondary objectives.
Objective
The aim of this report is to review and describe NORspine’s development, concepts, methods, cohort and future perspectives.
Development of NORspine
In 2000, the University Hospital of North Norway (UNN) established a single-institution database of consecutive cases operated in the lumbosacral spine. This was expanded to a government funded national registry, the NORspine, in 2007 (version 1.0). The establishment was supervised by a steering committee (advisory board from 2015) consisting of neurosurgeons and orthopaedic surgeons representing the four health regions and the national societies for neurosurgery, orthopaedic surgery and spine surgery.
All Norwegian hospitals providing spine surgery signed data processing agreements. This allowed encrypted online transfer of baseline data through the Norwegian Health Network, or alternatively, through a virtual private network (VPN) to a central data administration unit.
At 3- and 12-month follow-up, all reporting was questionnaire-based, and from 2009 (version 2.0) managed by NORspine’s administration without any involvement of the treating hospitals. Follow-up at 24 months was omitted because a study from the UNN database showed little health change but, a significant decrease in response rate from 12 to 24 months [14]. Patients responded at home, returning scannable questionnaires in pre-stamped addressed envelopes. Non-respondents received one reminder. From 2018, all patients received an additional short message service (SMS) reminder about returning the questionnaire. In April 2022, an electronic solution (ePROM) was introduced as the main follow-up routine [15].
To obtain adequate data for quality improvement and research, NORspine’s initial priority was data collection and validation. Clinical data were available online for each hospital through interactive and continuously updated reports, which from 2013 included benchmarking. Comprehensive annual reports were published from 2010. National quality indicators with benchmarks were established and revised annually. These enable comparison of results between hospitals and against aggregated national figures.
NORspine version 3.0 was launched in 2019 with revised questionnaires according to the International Consortium for Health Outcomes Measurement (ICHOM) recommendations [16]. Both patients’ and surgeons’ questionnaires were expanded with new variables. The consent form and the questionnaires were available in Norwegian only until English versions became available from 2022 (Online Resource 1, 2, 3, and 4).
NORspine’s methods
Study design
NORspine can be regarded a large population-based study comprising a national cohort. This paper is therefore reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for cohort studies [17].
We report baseline data for cases operated until December 31, 2021, follow-up data for cases operated until December 31, 2020 and the methods used and administrative development until December 31, 2022.
Setting
Public Norwegian healthcare is a single-payer tax-based system with universal access. Holding a private health insurance is uncommon (10% of the population in 2017) [18]. Healthcare is free of charge with a small co-payment (annual cap for 2022; EUR 270). Public hospitals are organised under the Ministry of Health and Care Services’ four regional health authorities. Both public and private hospitals provide spine surgery. Private hospitals treat patients with private insurance and patients who pay out of pocket. They may also contract with a regional health authority and provide publicly funded care for defined time periods.
Organisation and daily operations
The registry is a unit under the neurosurgical department at the UNN, led by an academic director (20%) and staffed with a coordinator/administrative lead (100%), a secretary (50%), and a professor (10% position). Ph.D. students and researchers with external funding from non-commercial sources may be affiliated for specific projects. The National Service Environment for Clinical Quality Registries provides advice on legal issues and statistical support for surveillance of data quality and reporting. The Northern Norway Regional Health Authority ICT Trust is IT vendor and developer. They collaborate with the Norwegian Health Network who coordinates access to secure e-health solutions.
At reporting units, a designated employee, preferably a spine surgeon, is responsible for data processing, while coordinators (usually a nurse or secretary) collect paper-based baseline questionnaires and consent forms, and punch data.
The advisory board guides data collection, management, analysis, and presentation. They define quality indicators, initiate quality improvement measures, direct research, process applications for data access and approve annual reports before publishing.
An annual open meeting gathers the register administration, the advisory board, local coordinators and surgeons, patients’ representatives, and any other stakeholders.
Funding
Funding is through the Northern Norway Regional Health Authority. NORspine receives no additional funding from industry or other stakeholders. The operating budget was EUR 242 000 in 2022. This included all labour and running costs for the registry administration. Costs for support from the National Service Environment for Clinical Quality Registries and the Northern Norway Regional Health Authority ICT Trust are embedded in a common budget for services to numerous other registries, and not possible to estimate specifically for NORspine.
User involvement
User involvement is coordinated by the Norwegian Spine Patients Association (Ryggforeningen). Their president is a member of the advisory board. The association facilitates information about and feedback on reports, findings, and recommendations through publications and meetings for users. They also organise user involvement in research- and improvement-projects.
Register cohort
All consecutive patients operated in public and private hospitals for degenerative disorders in the lumbosacral spine are eligible, with exception of patients precluded from consenting because of age < 16 years, severe psychiatric disorder, substantial drug abuse, cognitive impairment, or language barriers. Patients operated for tumours, trauma, primary infection or non-degenerative scoliosis are not eligible.
A reoperation within 90 days is registered as a complication to the index operation and not as a new case. Contrary, another operation after ≥ 90 days is considered a new case, and previously scheduled follow-ups are replaced by new follow-ups, 3 and 12 months after the latest operation.
Participation is voluntary and requires informed consent. Patients have a legal right to access, correct, or delete their data at any time [19]. For providers and healthcare personnel, giving information to eligible patients and reporting to national clinical quality registries became a legal obligation in 2019 [19]. Partaking is free and supported by a small reimbursement for providers (in 2022 EUR 18) for each registered case.
Data collection and variables
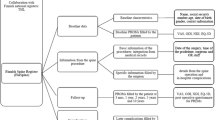
NORspine uses a set of baseline-, process- and outcome-variables recommended by the ICHOM [16]. Data are recorded prospectively by patients, surgeons, and local coordinators. Figure 1 shows the data collection process and Table 1 an overview of the variables. The main outcome measure is the Oswestry Disability Index (ODI) at 12-month follow-up. Important secondary outcomes are EuroQol 5 Dimensions 5 Levels (EQ-5D-5L), numeric rating scales (NRS) for back- and leg-pain, and the Global Perceived Effect (GPE) scale.
Patients report sociodemographic, health- and disease-related data such as comorbidities, previous treatments, waiting times and patient-reported outcome measures (PROMs) at baseline. At follow-ups, they report PROMs, patient-reported experience measures (PREMs), and postoperative complications. NORspine has established a possibility for re-contacting sub-groups for long-term follow-ups, e.g. for research purposes. This requires supplemental external funding.
Surgeons report data about clinical and radiological diagnosis(es), relevant comorbidity, previous spine surgery, clinical examination findings, type and duration of surgery and intraoperative complications. Based on all these variables, a validated algorithm (available upon request) defines main diagnosis and treatment (type of surgery) categories.
The main categories can be drilled down to more granular subcategories, based on information from the questionnaire (Online Resource 4). Since many diagnoses and treatments coexist, the system is hierarchical. This means that for each case, the most comprehensive type of surgery will define only one of eight unique main treatment categories (e.g. fusion surgery). Next, these are divided into 18 more specified subcategories (e.g. type of fusion technique). These can be further subclassified if additional procedures have been performed (e.g. different types of decompression). A corresponding logic has been established for categorization of the diagnoses. This enables definition and analysis of sub-groups of cases defined by specific combinations of diagnoses and treatments at an increasingly granular level (example in Online Resource 5). This also means that diagnosis and treatment categories (which may change over time) are dynamic and can be redefined, since they are not predefined on surgeons’ forms in fixed categories.
At discharge, local coordinators report the length of hospital stay and perioperative complications, including death.
Follow-ups apply the electronic reporting system ePROMs [15]. This is a generic solution for collection of patient-reported data for Norwegian clinical quality registries. Electronic questionnaires are mailed through the Norwegian Health Network or a public digital postal service (Digipost). More than 80% of the population are users [20].
Patients get a notification (by e-mail, short message service (SMS), or in the Digipost app, according to individual settings). They complete and return the questionnaire electronically to NORspine’s database. Non-respondents (after two digital inquiries) and digitally inactive patients are contacted by ordinary post according to previous routines. Thereafter, one more reminder is sent to non-respondents. Integration with the National Population Register assures continuous updating of addresses, and that deceased patients are not invited to follow-ups. In addition, this enables calculation of mortality rates.
Data quality
Completeness
Completeness is the extent to which all data that could have been registered, are recorded [21]. Coverage rate (at provider level), capture rate (at case level), response rate at follow-ups, and datapoint completeness describe this attribute.
The Norwegian Patient Registry (NPR) records the International Classification of Diseases, 10th revision (ICD-10) codes, and the Nordic Medico-Statistical Committee (NOMESCO) Classification of Surgical Procedures (NCSP) codes for all publicly funded specialist health care. Reporting is prerequisite for reimbursement. Standardised methods for calculation of the capture rate based on merging of NPR (denominator) and NORspine (numerator) data were developed in 2011, and rates were calculated back to 2008. Only cases with complete baseline datasets are included in these analyses. Capture rate analyses are routinely repeated biannually. Three studies [4, 7, 22] applied this method to calculate age- and sex-adjusted surgical rates at national and provider level, and the rates were comparable to those found in NORspine´s analyses [23].
Timeliness
Data must be available in time for surveillance of quality and scheduling of follow-ups. NORspine therefore surveils time from the operation to completion and punching of data from the baseline questionnaires.
Accuracy
Accuracy expresses the extent to which registered data are true [21]. It is important to use instruments with high validity and reliability to obtain accurate measurements and decrease the risk of information bias [24].
The register´s software has embedded logical restrictions to prevent registration of false and obviously erroneous values, and flag outliers. In addition, logical checks to identify outliers and systematic errors are performed annually.
The PROMs are valid and reliable in a Norwegian setting [16, 25,26,27].
Relevance and comparability
The dataset is in line with the ICHOM [16] recommendations, which facilitates comparability with other register cohorts [28]. This is exemplified by three studies merging data with other Scandinavian registries [29,30,31] and one study comparing data with the Research Patient Data Registry in Massachusetts, USA [32]. Registration of all known important predictors for outcome facilitates case mix adjustment, prognostic factor research, and predictive modelling.
Uninterrupted data collection, classification of diagnoses, treatment categories and outcome measures since 2007 assure internal temporal comparability.
Data availability
All providers have real-time online access to their data for quality improvement purposes. They may subscribe for quarterly automatically generated PDF reports for benchmarking with other providers.
Patients also consent to their use of data for research purposes. This includes merging with electronic heath record (EHR) data, other Norwegian health registries, and spine registries in other countries. Patients also consent to be contacted beyond the scheduled follow-ups, enabling long-term follow-ups, and recruitment to NORspine-associated studies.
Researchers are welcome to submit applications for data. Until 2022, NORspine had contributed to 73 peer-reviewed international publications.
Quality reporting
Benchmarking
A minimal clinical important change (MCIC) is the smallest improvement on an outcome scale being perceived as beneficial by a patient [33]. An expert panel workshop which based it’s assessment on a literature review suggested a MCIC cut-off for ODI at 10 points reduction from baseline [34].
Instead of reporting MCIC, it is recommended to report substantial improvements for benchmarking [35]. This is to ensure that differences are perceived as truly beneficial, reflecting treatment success for the patient. Such criteria can be used to compare surgical effectiveness, both temporally and between surgical departments or interventions [33, 36]. In contrast to MCIC, a success criterion can serve as a more ambitious and motivating benchmark for clinicians and registries aiming to improve quality. The GPE scale assesses the patient’s global perceived effect of the operation on a balanced 7-point Likert scale, from completely recovered to worse than ever. Accordingly, NORspine has developed GPE-anchored criteria for classification of outcomes [36,37,38,39].
Quality indicators
The advisory board now recommends five quality indicators (QI); three process- and two outcome measures. Seven former QIs were omitted in 2022 because their objective was achieved or they had overlapping purposes with other QIs. Table 2 summarises present and former QIs and presents aggregated national results for 2021.
The process indicators are evidence based and the outcome indicators aim to motivate surgeons to find measures to increase the proportion of cases with successful outcomes. Among patients operated for LDH and LSS, long duration of symptoms and minor leg pain are predictors for unfavourable outcomes [40, 41].
Data handling and analysis in this report
We retrieved a dataset containing all cases registered between 1 January 2007 and 31 December 2021. For 15,943 cases scored with EQ-5D-5L, we used a 5L crosswalk to derive corresponding EQ-5D-3L values [42].
Considering the central limit theorem, means of continuous variables were presumed to be normally distributed due to the large sample size. We present descriptive data as means with standard deviations (SD) or 95% confidence intervals (CI) and counts with percentages for proportions. Mean difference between groups were analysed with two-way students t-test for continuous variables and Pearson’s chi-squared test for categorical variables. The statistical significance level was set to p < 0.05. We used the Statistical Package for the Social Sciences (SPSS; version 28 (IBM Armonk, NY: IBM Corp)) for statistical analysis.
Results
Data quality
Completeness
The coverage rate has been stable at 100% for public and private providers since 2016.
Analyses based on comparison with the NPR showed that the number of cases and the capture rate increased gradually to 5980 and 74% in 2021 (Fig. 2) [23]. A dropout analysis revealed incomplete reporting of emergency surgery, especially during weekends and holidays, while the capture rate for elective surgery was 76% [23]. A supplemental analysis in 2021, which also included cases operated without public funding at private hospitals, indicated a total capture rate of 81% [23].
Published studies report response rates at 12-month follow-up ranging from 65 to 85% in 9 different sub-sets of the cohort [36,37,38, 43,44,45,46,47,48]. Internal audits of the total cohort show stable response rates around 70–75% [49]. A recent re-catch study [50] of 474 cases operated at four hospitals found a rate of 70%. Non-respondents were younger and more frequently smokers than respondents, but there were no differences in outcomes between the groups. An older study [14] found a response rate of 78%, and the non-respondents were younger, had shorter hospital stays, and were more likely to live alone. Non-respondents had fewer complications, but there were no differences in PROMs between groups.
Datapoint completeness is evaluated annually, and the mean was found stable above 97% for important variables [23].
Timeliness
Time from the operation to completion of the baseline questionnaires is satisfactory (median 1 and 0 days for patients’ and surgeons’ questionnaires, respectively) [23]. In 2021, 53% of the baseline data were punched and available within 4 weeks. Contrary, 7.5% of the cases were unavailable for 3-month follow-up due to delayed registration at baseline [23].
Accuracy
An audit in 2010 revealed error rates of 0.3% during punching of data and 0.04% after scanning of forms [23].
In 2012, a study re-examined magnetic resonance images (MRI) of 178 cases registered with lumbar disc herniation (LDH) and confirmed the diagnosis at the operated level in all [51]. A similar study from 2016 re-assessed MRIs of 202 cases registered with lumbar spinal stenosis (LSS) and confirmed the diagnosis for all [43]. In 2022, a study compared baseline data in the EHR at four public hospitals with data in NORspine [52]. It showed strong to excellent agreement for perioperative details (kappa ranging from 0.76 to 0.98) and excellent for patient-reported variables (kappa ranging from 0.93 to 0.99) [52]. The study reported weak agreement for surgeon-reported complications (kappa = 0.51) and moderate for The American Society of Anesthesiologists Physical Status Classification System (ASA classification) (kappa = 0.73).
An internal audit compared data in EHRs to NORspine’s algorithm-based classifications of treatments (types of surgery), and found only 3% misclassification [53].
Benchmarks
NORspine has conducted several GPE-anchored receiver operating curve analyses [33] to define criteria for success, failure and worsening at 12-month follow-up [36,37,38,39]. Table 3 shows these benchmarks, which are part of the routine reporting from the registry. As an example, the figure in Online Resource 6 shows boxplots of the ODI change stratified by GPE outcome categories, and relative to the MCIC and NORspine’s success criterium for patients operated for LDH.
The cohort
Table 4 shows an overview of the cohort’s baseline characteristics.
Until 31 December 2021, 60,647 cases were registered. 28,865 (47.6%) were females and the mean age was 55.7 years. The proportion > 70 years increased from 12.0% in 2008 to 26.1% in 2021 and the proportion with ASA classification > II from 9.9% in 2012 to 19.3% in 2021 (Fig. 3).
Mean scores (SD) for the PROMs at baseline was ODI 43.0 (17.3); NRS for leg pain 6.7 (2.3); NRS for back pain 6.5 (2.3); and EQ-5D-3L 0.35 (0.33).
Of the total cohort, 26,557 (43.8%) and 26,545 (43.8%) were operated with decompressive surgery for LDH and LSS, respectively. Among cases operated for LSS, 4002 (15.1%) had additional lumbar degenerative spondylolisthesis (LDS).
Totally, 7417 (12.2%) had undergone fusion surgery. In this group, 3739 (50.4%) had LSS (of which 1 385 (37.0%) had additional LDS), and 1383 (18.6%) had isthmic spondylolisthesis.
For cases registered until the end of 2020, the response rate was 71.8% at 3- and 71.6% at 12-month follow-up.
Respondents and non-respondents
Table 5 provides an overview of differences in baseline characteristics between respondents and non-respondents at 12-month follow-up for all cases until 31 December 2020 (n = 54,644). All characteristics, except perioperative complications, were statistically significantly different between the groups.
The respondents were older than the non-respondents, with a mean age of 57.7 (95% CI 57.6 to 57.9) and 49.9 (95% CI 49.7 to 50.2) years, respectively. There were lower proportions living alone, receiving sickness benefits, being a current smoker and having anxiety and/or depression, and a higher proportion reporting any relevant comorbidity among the respondents compared with the non-respondents (Table 5).
Discussion
This report on NORspine’s development, methods and cohort illustrates that establishing a national register is a laborious time- and resource-demanding process. It was made possible by a few enthusiastic spine surgeons supported by national professional societies and funding from health authorities. Thereafter, continuous development and improvement have been necessary to ensure data quality, data management and reporting. During this phase, waning attention from the funding authorities has been a challenge.
Nevertheless, we show that NORspine now is a well-designed and comprehensive national clinical quality register for lumbosacral spine surgery. It contains continuously updated validated data of acceptable quality on a large national cohort of cases.
Since the initiation in 2007, patients’ age and the proportion with ASA classification > II have increased. This indicates that spine surgeons are faced with more resource-demanding and complex cases, due to an ageing and frailer patient population.
Interpretation
Other spine registries
In 2002, Arts et al. suggested a generic framework for the organisation of and data quality in medical registries [21]. Drolet and Johnson detailed the requirements in 2008 by suggesting that registries must contain mergeable (M) and standardised data (D) collected according to specific rules (R) and that cases should be observed (O) over time for knowledge (K) about outcomes (the MDR-OK framework) [54]. Van Hoof et al. specifically reviewed the literature about spine registries in 2015, and developed recommendations to improve the quality of studies from such registries [8]. In addition to adherence to the MDR-OK, they recommended that registries should incorporate strategies to improve the quality of care, follow-up for at least one year, register the prognostic factors recommended by ICHOM to enable case-mix adjustments and link with EHRs to avoid double data entry. NORspine was organised and developed in accordance with these requirements, and now complies with all recommendations except integration with the EHR.
The design of NORspine, Swespine and DaneSpine are similar [9, 10, 23, 55, 56]. One difference is that NORspine requires informed consent, while Swespine and Danespine have opt-out legal regulations. This may contribute to the difference in capture rates, which were 81% in NORspine and 86% in Swespine in 2021 [23, 56]. In addition, NORspine’s capture rate is strictly defined, i.e. only cases with complete datasets, comprising both patients’ and surgeons’ baseline forms, are included. Importantly, the methods for calculation of capture rate might differ between spine registries, and should be documented.
Two studies compared cohorts operated for LDH and LSS across the three Scandinavian registries [29, 30]. They found that the gender distribution, mean age and BMI were similar at baseline. Otherwise there were only minor differences in baseline characteristics, which could easily be controlled for in pooled analyses [46, 47]. The Scandinavian countries comprise a relatively homogeneous population, and have similar social security and health care systems enabling merging and comparison of population-based data [29]. Still, the surgical rate for LDH in Sweden seems to be only half of those in Denmark and Norway [30]. Further standardisation of registry protocols across countries could improve the understanding of such discrepancies, and facilitate analyses of the potential impact of utilisation rates on outcomes.
Spine Tango, the American Spine Registry, and the Australian Spine Registry are based on institutional memberships [11, 12, 57, 58]. Optional participation for providers probably causes selection of institutions with a special interest in spine surgery or quality improvement, and capture skewed towards complex cases undergoing more specialised surgery. Therefore, the external validity of these registries is likely to be lower than for the population-based Scandinavian registries. They do, however, provide useful insights into other aspects, e.g. comparison of outcomes of specific procedures, when baseline characteristics are well described. This means that data merging across registries with different capture profiles requires careful case mix adjustment.
Strengths and limitations
NORspine assesses data quality by accuracy, completeness, relevance, timeliness and comparability [21, 23, 59]. It is a weakness that follow-up beyond 12 months (e.g. after 2 or 15 years), is possible only for projects with external funding to pay for the use of NORspine’s infrastructure.
Selection bias
Voluntary participation and recruitment based on informed consent ensure legitimacy in the public. However, self-selection could cause skewed capture and introduce attrition bias.
NORspine’s cohort has a lower proportion of cases undergoing emergency surgery than the target population. Patients having emergency surgery for LDH have greater clinical improvement than those undergoing scheduled operations [60]. Thus, interpretation of results should take this into account.
Until 2022, when English questionnaires became available, language barriers restricted inclusion to Norwegian speakers. Not being a native Norwegian speaker is associated with increased odds of failure after surgery for LSS [40]. Language barriers could impair the communication between surgeons and patients and cause less accurate selection for surgery. This means that if patients with language barriers are underrepresented, treatment effects in the total cohort could be overestimated.
Otherwise, little is known about possible bias introduced by incomplete capture. This could be studied by a more comprehensive comparison of data in NORspine and the EHR.
The Norwegian National Service Environment for Clinical Quality Registries has set the benchmark for capture rate at 80%. Like Prang et al. [59], we found no evidence for this recommendation, which may descend from the assumption that a response rate above 80% at follow-up is necessary to achieve a representative sample in cohort studies [61]. NORspine’s method for capture analysis has been peer-reviewed [4, 7, 22], indicating that the method is considered robust. Achieving a capture rate above 80% remains a goal, despite unclarity about it’s validity.
A review of spine registries recommended 12-month follow-up rate at 60 to 80% [8]. Re-catch studies of sub-cohorts in NORspine suggest that 27 and 22% loss to follow-up may not bias outcomes [14, 50]. This is in accordance with a similar study from DaneSpine and a study comparing Swespine data with data captured in a prospective observational study [62, 63]. These findings indicate that cases lost to follow-up are missing at random, implying loss to follow-up does not cause attrition bias if the variables associated with loss to follow-up are controlled for [61].
In contrast, Spine Tango recently compared 3-month outcomes of 12-month respondents with 12-month non-respondents [64]. They found that 12-month non-respondents had significantly worse early outcomes than respondents, and concluded that loss to follow-up as low as 11–14% may cause overestimation of the treatment effect. The authors speculated that re-catch studies introduce a social desirability bias. On the other hand, it is not obvious that 3-month data are an unbiased predictor of 12-month outcome. Also, Parai et al. [65] used regression models based on baseline characteristics of respondents to predict outcomes for non-respondents in Swespine. They predicted statistically significantly worse outcomes for non-respondents, but the differences were small.
It is a strength that public funding of NORspine prohibits influence from commercial stakeholders such as industry, which could increase the risk for publication bias.
Information bias
Use of predictors and validated and responsive PROMs recommended by ICHOM ensure accurate, relevant and comparable data, and is a strength [16, 25,26,27]. Indirectly, NORspine’s methods have also been validated through numerous peer-reviewed publications. However, continuous evaluation of data quality is mandated for any clinical registry to maintain legitimacy and financial support.
Even though the NORspine includes nearly all known predictors for outcome, residual confounding will exist. The PROMs have not been validated with qualitative studies for patients undergoing spine surgery, and they may not cover all aspects considered important by patients [66].
NORspine records early reoperations as a complication. Studies analysing administrative register data (NPR), assessing reoperation rates, comorbidity and complication rates [4, 22] found identical reoperations rates within 90 days, less comorbidity and lower complication rates in NPR compared to NORspine [23]. This indicates that reoperation rates are accurately reported, but comorbidity and complications seem to be underreported in administrative databases compared to NORspine [23]. Still, other studies indicate that complications and comorbidity tend to be underreported in registries, including NORspine, when compared to the EHR [52, 67]. In NORspine, surgeons record relevant comorbidity. They probably under-report because no clear definition of a relevant comorbidity exists. Quigley et al. recently reviewed comorbidity recorded in spine registries, including the completeness of such data, and how they are collected [68]. They reported significant methodological variation, and difficulties in assessing the completeness of the data. NORspine was one of few which collect comorbidity data directly from surgeons. Other registers collected such data from patients, the EHR or administrative databases.
Operational stability
Proper funding is mandatory to ensure sufficient staffing and sustainability. Due to limited funding, NORspine relies on a strong personal commitment from its staff. Especially, increased statistician support is warranted due to increasing demands for reporting, safe data management and delivery, and continuous data quality assessment.
Future perspectives
In 2023, NORspine launch a new register for cases operated for non-degenerative spine deformities and a register for spinal fractures might be a further expansion.
Register data are not readily available in everyday clinical practice. However, an innovation project which integrates NORspine and the EHR is in progress with piloting scheduled in 2024. This integration will aid in structuring the EHR, and availability of register data in the clinician’s regular user interface is expected to boost the capture rate. The project also integrates presurgical prognostic modelling of individuals’ probability for different surgical outcomes, by the use of artificial intelligence. The intention is to support shared decision-making at surgical outpatient clinics and to facilitate personalised health care and patient selection prior to surgery.
Currently, it is beyond of NORspine’s scope to record patients undergoing conservative treatment. Accordingly, we cannot assess whether surgery is offered to those who will benefit most. There could be undetected under- or over-supply in different regions, hospitals or sub-populations. Detecting unwarranted variation would require an expansion to capture all patients evaluated for surgery. A collaboration with the Norwegian Neck and Back Registry, a national clinical quality registry for non-surgical treatment at physical medicine and rehabilitation outpatient clinics, could potentially be a way forward. Spine Tango is, to our knowledge, the only surgical spine registry that also includes conservatively treated cases [57].
Generalisability
The NORspine is located in a country where access to health services is relatively uniform and government funded, and where the public trust to government is high. Some of these premises are unique for the Nordic countries. This limits the generalizability of health services research to countries with other health care systems.
Conclusion
NORspine is a well-designed population-based comprehensive national clinical quality registry. The register’s methods ensure appropriate data for quality surveillance and improvement, and research. Maintaining high data quality is a continuous and resource-demanding processes.
Data availability
Is restricted due to their sensitive nature. Anonymized data are available on reasonable request.
References
Brage S, Ihlebaek C, Natvig B, Bruusgaard D (2010) Musculoskeletal disorders as causes of sick leave and disability benefits. Tidsskr Nor Laegeforen 130:2369–2370. https://doi.org/10.4045/tidsskr.10.0236
Tollanes MC, Knudsen AK, Vollset SE, Kinge JM, Skirbekk V, Overland S (2018) Disease burden in Norway in 2016. Tidsskr Nor Laegeforen. https://doi.org/10.4045/tidsskr.18.0274
Gibson JN, Grant IC, Waddell G (1999) The Cochrane review of surgery for lumbar disc prolapse and degenerative lumbar spondylosis. Spine Phila Pa 1976 24:1820–1832. https://doi.org/10.1097/00007632-199909010-00012
Grotle M, Småstuen MC, Fjeld O, Grøvle L, Helgeland J, Storheim K, Solberg TK, Zwart JA (2019) Lumbar spine surgery across 15 years: trends, complications and reoperations in a longitudinal observational study from Norway. BMJ Open 9:e028743. https://doi.org/10.1136/bmjopen-2018-028743
Wennberg JE (2002) Unwarranted variations in healthcare delivery: implications for academic medical centres. BMJ 325:961–964. https://doi.org/10.1136/bmj.325.7370.961
Weinstein JN, Lurie JD, Olson PR, Bronner KK, Fisher ES (2006) United States’ trends and regional variations in lumbar spine surgery: 1992–2003. Spine Phila Pa 1976 31:2707–2714. https://doi.org/10.1097/01.brs.0000248132.15231.fe
Ingebrigtsen T, Balteskard L, Guldhaugen KA, Kloster R, Uleberg B, Grotle M, Solberg TK (2020) Treatment rates for lumbar spine surgery in Norway and Northern Norway Regional Health Authority 2014–18. Tidsskr Nor Laegeforen. https://doi.org/10.4045/tidsskr.20.0313
van Hooff ML, Jacobs WC, Willems PC, Wouters MW, de Kleuver M, Peul WC, Ostelo RW, Fritzell P (2015) Evidence and practice in spine registries. Acta Orthop 86:534–544. https://doi.org/10.3109/17453674.2015.1043174
Strömqvist B, Fritzell P, Hägg O, Jönsson B (2009) The Swedish spine register: development, design and utility. Eur Spine J 18(Suppl 3):294–304. https://doi.org/10.1007/s00586-009-1043-4
Simony A, Hansen KH, Ernst C, Andersen M (2014) Implementation of the Danish national database Danespine for spinal surgery. Ugeskr Laeger 176:V01130019
Apos E TT, Pickett C, Bulmer S, Ahern S, Cunningham J, Kuru R, Johnson MA (2022) Australian spine registry annual report, 2021.
Asher AL, Knightly J, Mummaneni PV, Alvi MA, McGirt MJ, Yolcu YU, Chan AK, Glassman SD, Foley KT, Slotkin JR, Potts EA, Shaffrey ME, Shaffrey CI, Haid RW, Fu KM, Wang MY, Park P, Bisson EF, Harbaugh RE, Bydon M (2020) Quality outcomes database spine care project 2012–2020: milestones achieved in a collaborative North American outcomes registry to advance value-based spine care and evolution to the American Spine Registry. Neurosurg Focus 48:E2. https://doi.org/10.3171/2020.2.FOCUS207
(2017) [Statute for The Norwegian registry for spine surgery]. In., Norway.
Solberg TK, Sorlie A, Sjaavik K, Nygaard OP, Ingebrigtsen T (2011) Would loss to follow-up bias the outcome evaluation of patients operated for degenerative disorders of the lumbar spine? Acta Orthop 82:56–63. https://doi.org/10.3109/17453674.2010.548024
Central Norway regional health authority's IT departments homepage for ePROMS (2022). https://eprom.hemit.org/. Accessed 23 June 2022
Clement RC, Welander A, Stowell C, Cha TD, Chen JL, Davies M, Fairbank JC, Foley KT, Gehrchen M, Hagg O, Jacobs WC, Kahler R, Khan SN, Lieberman IH, Morisson B, Ohnmeiss DD, Peul WC, Shonnard NH, Smuck MW, Solberg TK, Stromqvist BH, Hooff ML, Wasan AD, Willems PC, Yeo W, Fritzell P (2015) A proposed set of metrics for standardized outcome reporting in the management of low back pain. Acta Orthop 86:523–533. https://doi.org/10.3109/17453674.2015.1036696
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP (2008) The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 61:344–349. https://doi.org/10.1016/j.jclinepi.2007.11.008
Grepperud S (2018) [Privat medical treatment insurances—status and possible consequences for effectivity and allocation]. In. University of Oslo, University of Oslo
The Norwegian Ministry of Health and Care Services (2019) Forskrift om medisinske kvalitetsregistre [Regulation of medical quality registries]. https://lovdata.no/dokument/SF/forskrift/2019-06-21-789?q=medisinske%20kvalitetsregistre. Accessed 28 July 2023
(2021) [Trends 2021-eHealth trends]. In. Norwegian Directorate for e-health, https://www.ehelse.no/
Arts DG, De Keizer NF, Scheffer GJ (2002) Defining and improving data quality in medical registries: a literature review, case study, and generic framework. J Am Med Inform Assoc 9:600–611. https://doi.org/10.1197/jamia.m1087
Fjeld OR, Grøvle L, Helgeland J, Småstuen MC, Solberg TK, Zwart J-A, Grotle M (2019) Complications, reoperations, readmissions, and length of hospital stay in 34639 surgical cases of lumbar disc herniation. Bone Joint J 101(B):470–477. https://doi.org/10.1302/0301-620x.101b4.Bjj-2018-1184.R1
Solberg TK, Ingebrigtsen T, Olsen LR, Thyrhaug AM (2022) Årsrapport 2021: Nasjonalt kvalitetsregister for ryggkirurgi, resultater og forbedringstiltak [The Norwegian registry for spine surgery (NORspine). Annual report for 2021 with plan for improvement measures]. https://doi.org/10.7557/7.6865. Accessed 28 July 2023
Szklo M, Nieto FJ (2018) Epidemiology : Beyond the Basics. Jones & Bartlett Learning, LLC, Sudbury, UNITED STATES
Grotle M, Brox JI, Vøllestad NK (2003) Cross-cultural adaptation of the Norwegian versions of the Roland-Morris disability questionnaire and the oswestry disability index. J Rehabil Med 35:241–247. https://doi.org/10.1080/16501970306094
Solberg TK, Olsen JA, Ingebrigtsen T, Hofoss D, Nygaard OP (2005) Health-related quality of life assessment by the EuroQol-5D can provide cost-utility data in the field of low-back surgery. Eur Spine J 14:1000–1007. https://doi.org/10.1007/s00586-005-0898-2
Garratt AM, Furunes H, Hellum C, Solberg T, Brox JI, Storheim K, Johnsen LG (2021) Evaluation of the EQ-5D-3L and 5L versions in low back pain patients. Health Qual Life Outcomes 19:155. https://doi.org/10.1186/s12955-021-01792-y
Kaye ID, Butler JS, Morrissey PB, Sebastian AS, Wagner SC, Vaccaro AR (2018) Spine registries: Where do we stand? Clin Spine Surg 31:389–394. https://doi.org/10.1097/BSD.0000000000000589
Lønne G, Fritzell P, Hägg O, Nordvall D, Gerdhem P, Lagerbäck T, Andersen M, Eiskjaer S, Gehrchen M, Jacobs W, van Hooff ML, Solberg TK (2019) Lumbar spinal stenosis: comparison of surgical practice variation and clinical outcome in three national spine registries. Spine J 19:41–49. https://doi.org/10.1016/j.spinee.2018.05.028
Lagerbäck T, Fritzell P, Hägg O, Nordvall D, Lønne G, Solberg TK, Andersen M, Eiskjær S, Gehrchen M, Jacobs WC, van Hooff ML, Gerdhem P (2019) Effectiveness of surgery for sciatica with disc herniation is not substantially affected by differences in surgical incidences among three countries: results from the Danish, Swedish and Norwegian spine registries. Eur Spine J 28:2562–2571. https://doi.org/10.1007/s00586-018-5768-9
Andersen M, Fritzell P, Eiskjaer SP, Lagerbäck T, Hägg O, Nordvall D, Lönne G, Solberg T, Jacobs W, van Hooff M, Gerdhem P, Gehrchen M (2019) Surgical treatment of degenerative disk disease in three Scandinavian countries: An International register study based on three merged national spine registers. Global Spine J 9:850–858. https://doi.org/10.1177/2192568219838535
Lønne G, Schoenfeld AJ, Cha TD, Nygaard ØP, Zwart JAH, Solberg T (2017) Variation in selection criteria and approaches to surgery for Lumbar Spinal Stenosis among patients treated in Boston and Norway. Clin Neurol Neurosurg 156:77–82. https://doi.org/10.1016/j.clineuro.2017.03.008
Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, Haythornthwaite JA, Jensen MP, Kerns RD, Ader DN, Brandenburg N, Burke LB, Cella D, Chandler J, Cowan P, Dimitrova R, Dionne R, Hertz S, Jadad AR, Katz NP, Kehlet H, Kramer LD, Manning DC, McCormick C, McDermott MP, McQuay HJ, Patel S, Porter L, Quessy S, Rappaport BA, Rauschkolb C, Revicki DA, Rothman M, Schmader KE, Stacey BR, Stauffer JW, von Stein T, White RE, Witter J, Zavisic S (2008) Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 9:105–121. https://doi.org/10.1016/j.jpain.2007.09.005
Ostelo RW, Deyo RA, Stratford P, Waddell G, Croft P, Von Korff M, Bouter LM, de Vet HC (2008) Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine Phila Pa 1976 33:90–94. https://doi.org/10.1097/BRS.0b013e31815e3a10
Glassman SD, Copay AG, Berven SH, Polly DW, Subach BR, Carreon LY (2008) Defining substantial clinical benefit following lumbar spine arthrodesis. J Bone Joint Surg Am 90:1839–1847. https://doi.org/10.2106/jbjs.G.01095
Austevoll IM, Gjestad R, Grotle M, Solberg T, Brox JI, Hermansen E, Rekeland F, Indrekvam K, Storheim K, Hellum C (2019) Follow-up score, change score or percentage change score for determining clinical important outcome following surgery? An observational study from the Norwegian registry for spine surgery evaluating patient reported outcome measures in lumbar spinal stenosis and lumbar degenerative spondylolisthesis. BMC Musculoskelet Disord 20:31. https://doi.org/10.1186/s12891-018-2386-y
Alhaug OK, Dolatowski FC, Solberg TK, Lønne G (2021) Criteria for failure and worsening after surgery for lumbar spinal stenosis: a prospective national spine registry observational study. Spine J 21:1489–1496. https://doi.org/10.1016/j.spinee.2021.04.008
Solberg T, Johnsen LG, Nygaard OP, Grotle M (2013) Can we define success criteria for lumbar disc surgery? : estimates for a substantial amount of improvement in core outcome measures. Acta Orthop 84:196–201. https://doi.org/10.3109/17453674.2013.786634
Werner DAT, Grotle M, Gulati S, Austevoll IM, Lonne G, Nygaard OP, Solberg TK (2017) Criteria for failure and worsening after surgery for lumbar disc herniation: a multicenter observational study based on data from the Norwegian registry for spine surgery. Eur Spine J 26:2650–2659. https://doi.org/10.1007/s00586-017-5185-5
Alhaug OK, Dolatowski FC, Solberg TK, Lønne G (2022) Predictors for failure after surgery for lumbar spinal stenosis, a prospective observational study. Spine J. https://doi.org/10.1016/j.spinee.2022.10.010
Nygaard OP, Kloster R, Solberg T (2000) Duration of leg pain as a predictor of outcome after surgery for lumbar disc herniation: a prospective cohort study with 1-year follow up. J Neurosurg 92:131–134. https://doi.org/10.3171/spi.2000.92.2.0131
van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, Lloyd A, Scalone L, Kind P, Pickard AS (2012) Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 15:708–715. https://doi.org/10.1016/j.jval.2012.02.008
Weber C, Giannadakis C, Rao V, Jakola AS, Nerland U, Nygaard ØP, Solberg TK, Gulati S, Solheim O (2016) Is there an association between radiological severity of lumbar spinal stenosis and disability, pain, or surgical outcome?: A multicenter observational study. Spine (Phila Pa 1976) 41:E78-83. https://doi.org/10.1097/brs.0000000000001166
Austevoll IM, Gjestad R, Brox JI, Solberg TK, Storheim K, Rekeland F, Hermansen E, Indrekvam K, Hellum C (2017) The effectiveness of decompression alone compared with additional fusion for lumbar spinal stenosis with degenerative spondylolisthesis: a pragmatic comparative non-inferiority observational study from the Norwegian registry for spine surgery. Eur Spine J 26:404–413. https://doi.org/10.1007/s00586-016-4683-1
Werner DAT, Grotle M, Gulati S, Austevoll IM, Lønne G, Nygaard ØP, Solberg TK (2017) Criteria for failure and worsening after surgery for lumbar disc herniation: a multicenter observational study based on data from the Norwegian registry for spine surgery. Eur Spine J 26:2650–2659. https://doi.org/10.1007/s00586-017-5185-5
Werner DAT, Grotle M, Gulati S, Austevoll IM, Madsbu MA, Lonne G, Solberg TK (2020) Can a successful outcome after surgery for lumbar disc herniation be defined by the oswestry disability index raw score? Global Spine J 10:47–54. https://doi.org/10.1177/2192568219851480
Holmberg ST, Salvesen ØO, Vangen-Lønne V, Hara S, Fredheim OM, Solberg TK, Jakola AS, Solheim O, Nygaard ØP, Gulati S (2020) Pain during sex before and after surgery for lumbar disc herniation: a multicenter observational study. Spine Phila Pa 1976 45:1751–1757. https://doi.org/10.1097/brs.0000000000003675
Holmberg ST, Fredheim OMS, Skurtveit S, Salvesen ØO, Nygaard ØP, Gulati AM, Solberg TK, Gulati S (2022) Persistent use of prescription opioids following lumbar spine surgery: observational study with prospectively collected data from two Norwegian nationwide registries. Spine (Phila Pa 1976) 47:607–614. https://doi.org/10.1097/brs.0000000000004275
Solberg TKI, T, Olsen, L. R, Thyrhaug AM (2020) Årsrapport 2019: Nasjonalt kvalitetsregister for ryggkirurgi, resultater og forbedringstiltak [The Norwegian registry for spine surgery (NORspine). Annual report for 2019 with plan for improvement measures]. https://doi.org/10.7557/7.7184. Accessed 28 July 2023
Kaur S, Alhaug OK, Dolatowski FC, Solberg TK, Lønne G (2023) Characteristics and outcomes of patients who did not respond to a national spine surgery registry. BMC Musculoskelet Disord 24:164. https://doi.org/10.1186/s12891-023-06267-3
Sørlie A, Moholdt V, Kvistad KA, Nygaard ØP, Ingebrigtsen T, Iversen T, Kloster R, Solberg TK (2012) Modic type I changes and recovery of back pain after lumbar microdiscectomy. Eur Spine J 21:2252–2258. https://doi.org/10.1007/s00586-012-2419-4
Alhaug OK, Kaur S, Dolatowski F, Smastuen MC, Solberg TK, Lonne G (2022) Accuracy and agreement of national spine register data for 474 patients compared to corresponding electronic patient records. Eur Spine J 31:801–811. https://doi.org/10.1007/s00586-021-07093-8
Solberg TKO, LR Berglund (2019) Årsrapport for 2018 med plan for forbedringstiltak: Nasjonalt kvalitetsregister for ryggkirurgi [The Norwegian registry for spine surgery (NORspine). Annual report for 2018 with plan for improvement measures]. https://unn.no/Documents/Kvalitetsregistre/Nasjonalt%20kvalitetsregister%20for%20ryggkirurgi/%C3%85rsrapporter/%C3%85rsrapport_NKR_2018%20.pdf. Accessed 28 July 2023
Drolet BC, Johnson KB (2008) Categorizing the world of registries. J Biomed Inform 41:1009–1020. https://doi.org/10.1016/j.jbi.2008.01.009
Andersen MN, M; Bech-Azeddine, R; Helmig, P; Eiskjær, S (2021) Ryggkirurgi i Danmark Årsrapport 2021 [DaneSpine annual report 2021]. https://drks.ortopaedi.dk/wp-content/uploads/2022/05/%C3%85rsrapport-DRKS-2021-Godkendt.pdf. Accessed 28 July 2023
Fritzell P, Hägg O, Gerdhem PAA, Skarvinge C, Parai C, Thoreson O, Stömqvist B, Löfren H Mellgren L, Blom C (2022) Rapport 2022 - Swespine Årsrapport 2022 [Swespine annual report 2022]. http://www.4s.nu/Homepage/Download-File/f/1346053/h/7c5b48e6b00aee249aefba5b46a632b6/3.+220919_PF_Swespine_%C3%85rsrapport_2022_Ver15_SENT. Accessed 28 July 2023
Munting ET, Aghayev M, Sutter E, Armstrong, Swaby R, Caton O, E EUROSPINE Spine Tango International Report 2021
McGirt MJ, Speroff T, Dittus RS, Harrell FE Jr, Asher AL (2013) The national neurosurgery quality and outcomes database (N2QOD): general overview and pilot-year project description. Neurosurg Focus 34:E6. https://doi.org/10.3171/2012.10.FOCUS12297
Prang KH, Karanatsios B, Verbunt E, Wong HL, Yeung J, Kelaher M, Gibbs P (2022) Clinical registries data quality attributes to support registry-based randomised controlled trials: a scoping review. Contemp Clin Trials 119:106843. https://doi.org/10.1016/j.cct.2022.106843
Elkan P, Sjövie Hasserius J, Gerdhem P (2016) Similar result after non-elective and elective surgery for lumbar disc herniation: an observational study based on the SweSpine register. Eur Spine J 25:1460–1466. https://doi.org/10.1007/s00586-016-4419-2
Kristman V, Manno M, Cote P (2004) Loss to follow-up in cohort studies: How much is too much? Eur J Epidemiol 19:751–760. https://doi.org/10.1023/b:ejep.0000036568.02655.f8
Højmark K, Støttrup C, Carreon L, Andersen MO (2016) Patient-reported outcome measures unbiased by loss of follow-up. Single-center study based on DaneSpine, the Danish spine surgery registry. Eur Spine J 25:282–286. https://doi.org/10.1007/s00586-015-4127-3
Elkan P, Lagerbäck T, Möller H, Gerdhem P (2018) Response rate does not affect patient-reported outcome after lumbar discectomy. Eur Spine J 27:1538–1546. https://doi.org/10.1007/s00586-018-5541-0
Mannion AF, Fekete TF, O’Riordan D, Loibl M, Kleinstück FS, Porchet F, Reitmeir R, Jeszenszky D, Haschtmann D (2023) Does loss to follow-up lead to an overestimation of treatment success? Findings from a spine surgery registry of over 15,000 patients. Eur Spine J. https://doi.org/10.1007/s00586-023-07541-7
Parai C, Hägg O, Willers C, Lind B, Brisby H (2020) Characteristics and predicted outcome of patients lost to follow-up after degenerative lumbar spine surgery. Eur Spine J 29:3063–3073. https://doi.org/10.1007/s00586-020-06528-y
Calmon Almeida V, da Silva Junior WM, de Camargo OK, de Santana Filho VJ, Oliveira GU, Santana MS, de Farias Neto JP (2020) Do the commonly used standard questionnaires measure what is of concern to patients with low back pain? Clin Rehabil 34:1313–1324. https://doi.org/10.1177/0269215520941042
Ohrn A, Olai A, Rutberg H, Nilsen P, Tropp H (2011) Adverse events in spine surgery in Sweden: a comparison of patient claims data and national quality register (Swespine) data. Acta Orthop 82:727–731. https://doi.org/10.3109/17453674.2011.636673
Quigley M, Apos E, Truong T-A, Ahern S, Johnson MA (2023) Comorbidity data collection across different spine registries: an evidence map. Eur Spine J 32:753–777. https://doi.org/10.1007/s00586-023-07529-3
Acknowledgements
The authors thank secretary Mai Lisbet Berglund for contributing valuable information about NORspine’s history, daily operations and organisation, and for long-standing contributions to the continuous operation of the registry.
Funding
Open access funding provided by UiT The Arctic University of Norway (incl University Hospital of North Norway). This work was funded by a research grant from the Northern Norway Regional Health Authority (grant number HNF1538-20).
Author information
Authors and Affiliations
Contributions
Data collection and analysis, and writing of the first manuscript were performed by EM, TI and TS. All authors commented on previous versions and have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors are employees and/or members of NORspine’s advisory board. Otherwise they have no competing interests to declare.
Ethical approval
The Regional Committee for Medical and Health Research Ethics Northern Norway defined this report/study as quality improvement (file 369360) and the Data protection officer at the University Hospital of North Norway granted approval (file 02813).
Informed consent
Informed consent was obtained from all individual participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mikkelsen, E., Ingebrigtsen, T., Thyrhaug, A.M. et al. The Norwegian registry for spine surgery (NORspine): cohort profile. Eur Spine J 32, 3713–3730 (2023). https://doi.org/10.1007/s00586-023-07929-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-023-07929-5