Abstract
Introduction
This registry study aims to assess the prevalence and demographic characteristics of patients with lumbar spine (LS) surgical procedures who undergo total hip arthroplasty (THA), to compare the long-term survival and causes of failure of THA in patients who previously underwent LS fusion and non-fusion surgical procedures, and to evaluate the risk of undergoing a revision LS surgery after THA.
Materials and methods
Patients who underwent LS surgery followed by THA were identified by cross-referencing data from the Orthopedic Prosthetic Implants Registry and the Regional Hospital Discharge Database. Three groups of THA patients were identified: patients who underwent previous lumbar surgery with fusion (LS fusion-THA), without fusion (LS non-fusion-THA), and a control group with only THA (No LS surgery–THA). Demographic data, THA survival, number and causes of failure, and data on revision procedures on THA and LS were collected.
Results
Of the total of 79,984 THA, 2.2% of patients had a history of LS procedures. THA only patients showed better results, while patients in the LS fusion-THA group had worse implant survival at 5-year follow-up. In the LS fusion-THA and LS non-fusion-THA, mechanical THA failures were more frequent in the first two years after implantation. There were no differences between groups regarding the risk of undergoing LS revision surgery.
Conclusions
LS surgery negatively affects THA survivorship. In patients who previously underwent LS fusion and non-fusion surgical procedures, most THA failure occurs in the first two years after implant. The study contributes to the understanding of the relationship between the hip and the LS and provides useful guidance for clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ageing of the population, expansion of the indications, and reduction of invasiveness of surgery, together with the advancement in anaesthesiologic techniques that increase the eligibility of patients to major surgical procedures, led an increased number of patients to be subjected to both total hip arthroplasty (THA) and lumbar spine (LS) surgery during their lifetime [1].
THA is a successful surgery; however, it is associated with a non-negligible failure rate and a subsequent need for revision surgery [2]. THA failure is more frequent in particular subgroups of patients. Among these, patients with a history of LS diseases already subjected to LS surgery are prone to the development of a pathological pelvic version and abnormal spinopelvic kinematics [3,4,5] potentially responsible for dislocation, instability, and other mechanical complications of THA implants [6, 7].
Most available literature up to date regards the risk of developing complications in patients with previous LS fusion, in which the restricted spinopelvic motion and potentially altered pelvic version compromise the effective reciprocal coupling of the acetabular cup and prosthetic femoral head [8]. The mismatch of “coupled anteversion” can determine a reduced range of motion, altered biomechanics and poor tolerance towards inadequate positioning of the implant components, which justify the increase in mechanical complications of THA implants in patients operated on by LS fusion (LSF) [9,10,11].
A recent European study demonstrated that patients with THA and LS fusion showed an increased rate of mechanical complications [12], partly confirming data from US registries [6].
Conversely, little is known about the effects of non-fusion LS surgical procedures on THA survivorship [7, 13]. In the registry-based study on patients with both THA and LS surgery with data aggregated for fusion and non-fusion procedures, Eneqvist et al. [14] demonstrated that 3.5% of THA patients were subjected to LS surgery in the antecedent 11-year timeframe and that those patients generally had a poorer outcome of THA surgery. No study so far analysed the separated effects of LS fusion and non-fusion surgery on THA; moreover, assuming a two-way cause-and-effect relationship between THA and LS fusion, the effects of THA on survival of lumbar spine procedures meant the need for subsequent spine revision surgery after THA implant has been poorly explored so far.
Therefore, the aim of this registry-based population study is to assess the prevalence, the demographic characteristics and the long-term survival and causes of failure of THA in patients who previously underwent LS fusion and non-fusion surgical procedures, and to evaluate the risk of undergoing a revision LS surgery after THA.
To the authors' knowledge, this is the first registry study to evaluate THA survivorship in patients previously subjected to either LS fusion or non-fusion procedures. Moreover, it contributes significantly to the paucity of available data on LS surgery failure in patients subjected to THA.
Materials and methods
A registry-based retrospective comparative cohort study was performed to assess:
-
1.
The prevalence and the demographic characteristics of patients with LS surgical procedures (LS fusion and LS non-fusion) who underwent a subsequent THA surgery
-
2.
the long-term survival causes either mechanical and non-mechanical and timing of failure requiring revision surgery of THA implants in patients who previously underwent LS fusion (LS fusion-THA) and non-fusion procedures (LS non-fusion-THA), compared to THAs patients operated on in the same timeframe without previous LS surgeries (No LS surgery-THA);
-
3.
the long-term survival of LS procedures in patients undergoing subsequent THA surgery, intended as no need for revision spine surgery.
Patients subjected to LS surgical procedures and subsequent THA were identified by a cross-match of the Register of the Orthopaedic Prosthetic Implants (RIPO) and the Regional Hospital Discharge Form database of the Emilia Romagna Region. The study cohort included patients subjected to THA between 1 January 2000 and 31 December 2019 who previously had LS surgery procedures. This cohort was divided into THA patients who underwent LS fusion (LS fusion-THA) and THA patients who underwent non-fusion surgical procedures (LS non-fusion-THA). A control group consisted of patients subjected to THA only during the same time frame (No LS surgery-THA).
Founded in 1990, RIPO counts over 95% of all arthroplasty procedures performed in the region, including the hip. RIPO's data collection includes patient demographics and sex, clinical history, diagnosis, model and design of the implant, the surgeon performing the procedure, and hospital location. Similar information is recorded for patients residing in ERR for all revision surgeries, and it is obtained even if a patient receives revision surgery outside the region. THA procedures performed on patients residing outside ERR were excluded to minimise bias due to loss to follow-up and to match with LS surgery procedures patients.
Patients undergoing LS surgical fusion and non-fusion procedures were retrieved from the ERR Health System, querying the RHDF database for the same timeframe. Diagnostic-related group (DRG) codes were selected from ICD-9 related to spinal fusion and non-fusion procedures. DRG codes used for the study included primary LSF codes (81.04, 81.05, 81.06, 81.07, and 81.08) and revision lumbar fusion codes (81.34, 81.35, 81.36, 81.37, and 81.38). LS non-fusion surgery patients were identified using specific non-fusion codes (80.50; 80.51; 80.52; 80.59; 84.64; 84.65; 84.68).
Data enquiry for the registry and database was performed in July 2022. In the study timeframe (January 2000 to December 2019), 129,910 primary THAs were performed in ERR; only the first THA surgery was included. In the same timeframe, 113,698 LS surgery procedures were identified. Even in this case, only the first LS surgery was included. Of these, 79,984 THA and 65,129 LS surgical procedures were performed in patients residing in ERR. A crossover comparison between RIPO and RHDF databases was performed using the patient’s unique personal identification number. In total, 1744 patients underwent THA after an LS surgery.
Demographic data, primary diagnosis leading to THA, primary THA implant survival, perioperative complications, number and causes of failure, and data of revision procedures were collected. Implant survival was intended as the absence of revision events, which specifically refers to the removal or change of any component. The implants were followed until the last observation date (date of death or 31 December 2019). Data from 79,984 THAs performed in patients without a previous LS procedure in the same timeframe were used as a control for comparison.
Ethical approval for the study was not required as registry studies are covered by the informed consent signed at the point of treatment. All sensitive data were handled in a pseudo-anonymized format, with all patients’ identifiers being removed.
Statistical analysis
Patient demographics, preoperative diagnoses, and causes of revision were presented as percentages. Fisher's exact test was used to detect differences between cohorts in numbers and types of complications and the timing of complications. Continuous variables were compared between groups using independent samples t-test. The survivorship of the primary THA implants was calculated and plotted according to the Kaplan–Meier method. The study's endpoints were revisions for any reason and revisions due to mechanical complications (aseptic loosening, dislocation or instability, polyethylene wear, implant breakage and impingement, severe stiffness, and metallosis). The revision event was defined as the removal or change of any component. The implants were followed until the last observation date (date of death or 31 December 2019). Mann–Whitney U test was used to detect differences between the survivorship curves. The threshold for significance was p = 0.05 for all the tests. The statistical analysis was performed using SPSS 14.0 for Windows, version 14.0.1 (SPSS, Chicago, Illinois, USA) and JMP, version 12.0.1 (SAS Institute, Cary, North Carolina, USA).
Results
-
1) Prevalence and demographics
Of the 79,984 THA patients, 1744 patients underwent THA and previous LS surgical procedures (2.2%): 1,197 (1.5%) of THA patients underwent LS non-fusion surgery, while 547 (0.7%) underwent a LS fusion surgery. Patients’ demographics are summarised in Table 1.
50.1% of LS non-fusion-THA were males and showed an average age at surgery of 66.9 years. LS fusion-THA patients were males in 37.7% of cases and showed an average age at surgery of 68 years. No LS surgery-THA patients were males in 39.3%, having an average age at surgery of 68.8 years. LS non-fusion-THA patients were significantly younger and more frequently males than others. In all groups, the primary indication for THA implant was primary hip osteoarthritis; the second leading causes were fractures and trauma (Table 1).
Implants’ characteristics in the study population are reported in Table 2. Patients operated on at the lumbar spine were more often implanted with a head diameter of 32 or 36 mm (p< 0.05; chi-squared test). Most patients had a standard stem implant, while neck preservation implants were used in only a minority of the three groups.
-
2) Long-term survival, causes and timing of failure of THA in patients who previously underwent LS
No LS surgery-THA patients showed better implant survival than the other groups. Conversely, LS fusion-THA patients showed a higher rate of failures at long-term follow-up (p < 0.05, Wilcoxon test) (Fig. 1). Considering specific time points, at five years FU, the survival rate of No LS surgery-THA patients was 96.7% (95% CI 96.6–96.9); LS non-fusion-THA patients achieved a 96.1% (95% CI 94.6–97.1) survival rate, while LS fusion-THA patients achieved a 94.6% (95% CI 91.9–96.4) survival rate. At 15 years FU, the No LS surgery-THA group achieved a survival rate of 90.7% (95% CI 90.3–91.0), whereas 88.7% of LS fusion-THA (95% CI 76.8–94.9) and 87.7% (95% CI 81.9–91.8) of LS non-fusion-THA implants survived at 15 years.
The causes of the failure of THA in the three groups are reported in Table 3. In the No LS surgery-THA group, a total of 3980 failures leading to revision surgery were found, of which 1462 (36.7%) occurred in the first two years after surgery, 771 (19.4%) occurred between the third and the fourth year, and 1747 (43.9%) occurred after the fifth year. LS non-fusion-THA group counted a total of 63 failures, 31 (49.2%) in the first two years, 10 (15.9%) between the third and fourth year, and 22 (34.9%) after 5 years. LS fusion-THA group showed 27 failures, 18 (66.6%) in the first two years, six (22.2%) between the third and fourth year, and three (11.1%) at five or more years. Table 4 shows details about the timing of complications.
Mechanical complications accounted for 63.9% of the total cause of failure, 57% in LS non-fusion-THA, 63% in LS fusion-THA, and 64% in the No LS surgery-THA patients. The survival rate considering a mechanical complication as an endpoint showed significant differences between groups (Fig. 2). In particular, No LS surgery-THA and LS non-fusion-THA groups showed better survival than the LS fusion-THA group in the first five years (p = 0.05).
Significant differences were noted comparing the timing of failure in different groups, with LS surgery patients (fusion and non-fusion) presenting a higher incidence of failure within the first two years (p = 0.002) (Table 4). When only mechanical complications were considered, including mechanical loosening, implant dislocation, implant breakage, polyethene wear, primary instability and metallosis [12] that caused recurrent dislocation, impingement, or severely reduced range of motion which required revision surgery, a similar pattern was found (p = 0.004) (Table 5).
When the risk of instability or dislocation was assessed with respect to the size and type of head and cups (Table 6), it was found that among any LS surgery patients, those with heads ≤ 28 mm showed a 3.2 (1.1–9.2) times higher risk of revision for instability or dislocation compared to head diameters of 32 (p = 0.03), and 4.1 (1.4–11.9) times higher risk compared to those with 36 mm heads (p = 0.01). No LS patients operated of THA with dual mobility cup implant was revised for instability or dislocation at follow-up; however, due to the number of implants, no significant protection from the risk of dislocation was detected. When the type of stem was considered, two cases of instability or dislocations occurred when neck preservation implants were used.
-
3) Risk of undergoing a revision spine surgery after THA performance
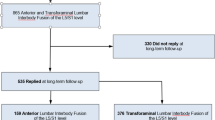
Considering patients who underwent LS surgery and subsequent THA, 91 (5.2%) required revision LS surgery (either fusion or non-fusion). Of those, 62 patients (5.2% of the total population group) were part of the LS non-fusion-THA group, while 29 patients (5.3% of the total population group) belonged to the LS fusion-THA group. No significant differences were found between LS fusion and non-fusion patients undergoing THA on the risk of performing another LS surgery (p = 0.379; Wilcoxon rank test). The Kaplan–Meier curves are presented in Fig. 3.
Discussion
In the current registry-based retrospective comparative study, we found that 2.2% of THA patients were subjected to previous LS surgery procedures. Of these, less than half were LS fusion surgeries. Analysis of THAs survival showed that No LS surgery-THA patients had a better THA implant survival than patients subjected to LS procedures, with LS fusion-THA patients showing a significantly higher risk of undergoing THA revision surgery. Interestingly, considering only mechanical complications as the endpoint for THA survival, the LS non-fusion-THA group showed better survival than the LS fusion–THA group in the first five years after surgery. Noteworthy, almost 80% of the total mechanical failures (and almost 70% of total failures) occurred significantly more in the first two years after primary THA surgery in patients who underwent previous LS surgery procedures. As regards the risk of undergoing LS revision surgery after THA, no differences were found between patients with LS fusion and non-fusion procedures [15].
The main limitation of the current study is the retrospective nature of the investigation, and the enrolment is limited to the population residing in ER due to the intrinsic nature of the RIPO registry. The lack of clinical and radiological data and information about minor complications that did not lead to THA revision surgery may underestimate patients’ disabilities in particular subgroups. Moreover, it was impossible to obtain information from the LS surgery on the detail or complexity of the procedures, including the number of fused levels, the inclusion of sacrum or pelvis in the fusion, the use of instrumentation and/or the use of bone graft. However, compared to current literature, the introduction of LS non-fusion-THA patients gives a unique insight into a population that has been understudied so far, allowing a combined registry analysis and comparison of LS fusion and non-fusion surgery patients undergoing THA.
LS non-fusion-THA patients were younger and with a higher number of males compared to the other groups. Even though this finding does not appear clinically relevant, it confirms the trend observed in current literature in which non-fusion LS surgery is mainly performed in younger patients compared to those undergoing fusion procedures [16, 17] because youngsters accept the risk of revision surgery to avoid early segmental LS fusion. Apart from patients’ age and weight, the main difference among groups was the male to female ratio: in the No LS surgery-THA group, males were 39.3%, similarly to LS fusion-THA (37.7%), while in the LS non-fusion-THA group they accounted for 50.1%. These data are in line with similar studies comparing patient demographics in LS surgery [17].
Different studies emphasise the correlation between THA and prior or subsequent LS fusion surgery [18, 19]; there is a consensus to perform THA before LS fusion surgery in patients complaining from both hip and LS diseases because THA could improve spinopelvic imbalance by promoting pelvic retroversion [1, 10, 20,21,22], also decreasing the risk of implant dislocation [22]. In a meta-analysis, An et al. [23] demonstrated that patients with a prior LSF showed a significantly higher complication rate following THA, including a twofold higher risk of dislocation and a threefold risk of undergoing revision arthroplasty surgery. Prior lumbar fusion was associated with poorer Patients Reported Outcome Measures (PROMs) following THA.
Our data agree with this observation since patients with fusion at the lumbar spine tended to show an increased incidence of dislocation (1.5% vs 0.7%) and instability (0.4% vs 0.1%) compared to patients subjected to THA alone. Even this is not available from our data, most probably patients with multilevel fusion and those whose fusion extends to the sacrum or pelvis might be stiffer and at risk for revision surgery for instability or dislocation compared to patients with single-level fusion or with a fusion not including sacrum or pelvis [19].
Overall, we found reduced THA implant survivorship in patients already subjected to fusion or non-fusion LS surgery. Lumbar spine motion's absent or reduced protective effect and an altered pelvic version may account for this result. Moreover, the restricted spinopelvic motion, abnormal pelvic version and acetabular anteversion compromise the reciprocal coupling of the cup and the stem, determining a poor tolerance to inadequate functional positioning of the prosthetic components [8, 12, 24,25,26]; this altered joint biomechanics may justify the increased mechanical complications observed in the study population.
The current study agrees with recent literature demonstrating a compromise in the survival of THA implants depending on stiffness or deformity of the lumbar spine and pelvis [27,28,29]. Indeed, LS non-fusion patients presented better THA implant survival compared to the LS fusion group, above all when biomechanical complications leading to revision surgery were considered. To decrease the risk of dislocation, cup anteversion should be carefully adjusted considering the functional position of the pelvis; moreover, the use of higher diameter heads, increased offset stems and use of dual mobility cups may further increase the range of motion while reducing the risk for of instability and dislocation [26, 27]. Interestingly, no LS surgery patient operated on by dual mobility cup implants was revised for instability or dislocation in our study cohort, supporting the use of those implants in LS patients undergoing THA. Moreover, LS patients with head diameters of 32 or 36 mm showed a reduced risk of revision surgery for revision or dislocation or instability compared to those with heads of 28 mm and under. The study revealed an increased implant of 28 mm diameter heads among THA patients without previous LS surgery. This finding reinforces the main findings of the study, namely that patients with LS surgery are more predisposed to mechanical complications of THA despite the increased use of larger diameter heads. This observation reflects the widespread utilisation of 28 mm heads, particularly during the initial half of the 2000–2010 decade. The group of patients with THA only, due to its larger size, better reflects this phenomenon, compared to LS fusion and non-fusion-THA patients. We also trust that a significant portion of patients who had previous LS surgery was intentionally treated by implant of large diameter heads.
Temporal evaluation of THA failures highlighted that THA patients also subjected to LS surgical procedures showed significantly more complications in the first two years after THA surgery. This finding was observed in both LS fusion and non-fusion patients. These results are consistent with current literature regarding the timing of complications leading to revision surgery [19, 22, 23, 25]. Most probably, the mutual coupling of the prosthetic components is impaired from the very beginning after THA in patients who underwent LS surgery, particularly when fusion procedures are performed. Conversely, the potentially intact flexibility of the LS region in the control group may promote a more effective reciprocal coupling of THA components. However, this protective mechanism may deteriorate over time because of the progressive physiological stiffening of the LS spine, sagittal imbalance, and altered pelvic version occurring with age, which might be responsible for the distribution of mechanical complications throughout the observation period in No LS Surgery-THA patients.
As regards the risk of undergoing revision LS surgery after THA, the literature is scarce in this regard, presenting only a few studies which show that LS surgery, in particular fusion, is potentially detrimental if performed on patients with stiff hips [18, 19, 23]. In the study by Pizones et al. [29], it was highlighted that during bipedalism, pelvic motion is necessary to maintain adequate balance and sagittal alignment. That retroversion is used as a compensatory mechanism when spinal malalignment occurs. Mills et al. [20] analysed patients who underwent THA after LS fusion and showed an increased rate of lumbar-related complications with respect to patients who did not undergo THA or to those who underwent THA before LS fusion; in particular, these patients were at increased risk to develop a failed back surgery for the development of adjacent segment disease at the lumbar spine. The current study, for the first time, analyses the potential role of THA on the risk of developing a failed back requiring revision LS surgery. Our results showed that THA surgery performance similarly affected patients subjected to fusion or non-fusion LS procedures. Unfortunately, it was impossible to appropriately weigh the role of THA surgery because of the lack of an adequate control group, namely patients subjected to LS surgery but not THA.
It is still a topic of debate how the pathological entities affecting the hip and spine interact, especially following surgery [30]. Given the strong functional relationship between the hip and lumbar spine, it is conceivable that surgery at one of the two segments influences the evolution of the other. Based on current literature, there is reason to suppose that THA patients are negatively affected by LS surgery [2, 6, 11, 31]. However, it is not the operation that compromises the THA's survival, but rather the pathology of the spine that defines the surgical indication and the postoperative overall sagittal balance of the patient. Indeed, even a multilevel fusion carried out on a rigid and unbalanced spine, performed according to the most recent recommendations regarding sagittal balance [1], can potentially improve spinopelvic relationships and function, not compromising or potentially improving THA survival in patients with LS disease.
Although the present study did not demonstrate it, the same can be potentially said about the effect of THA on the survival of LS surgeries: when a patient is given an indication for THA, a significant reduction in range of motion with possible hip flexion contracture can be found, and it usually improves following THA, promoting hip compensatory mechanisms and potentially a better sagittal balance of the spine [1, 32, 33].
In conclusion, our study confirms that LS surgery is performed in a small fraction of THA patients. However, this subgroup is associated with more mechanical complications, which can lead to early THA revision. These failures are significantly more frequent in the first two years after THA, thus defining a very vulnerable time period worthy of attention from the surgeon. Both LS fusion and non-fusion patients present a comparable risk of failed back following THA, necessitating surgical revision of the LS region. These findings contribute to the knowledge of the hip-spine relationship and may give helpful advice to surgeons performing spine or hip surgery.
References
Haffer H, AdlAmini D, Perka C, Pumberger M (2020) The impact of spinopelvic mobility on arthroplasty: implications for hip and spine surgeons. J Clin Med 9(8):2569. https://doi.org/10.3390/jcm9082569
Kelmer G, Stone AH, Turcotte J, King PJ (2021) Reasons for revision: primary total hip arthroplasty mechanisms of failure. J Am Acad Orthop Surg 29(2):78–87. https://doi.org/10.5435/JAAOS-D-19-00860
Rebeyrat G et al (2022) Assessment of dynamic balance during walking in patients with adult spinal deformity. Eur Spine J 31(7):1736–1744. https://doi.org/10.1007/s00586-022-07199-7
Mekhael E et al (2023) Functional assessment using 3D movement analysis can better predict health-related quality of life outcomes in patients with adult spinal deformity: a machine learning approach”. Front Surg. https://doi.org/10.3389/fsurg.2023.1166734
El Rachkidi R et al (2022) Spinopelvic adaptations in standing and sitting positions in patients with adult spinal deformity. Cureus. https://doi.org/10.7759/cureus.28113
Onggo JR et al (2020) Clinical outcomes and complication profile of total hip arthroplasty after lumbar spine fusion: a meta-analysis and systematic review. Eur Spine J 29(2):282–294. https://doi.org/10.1007/s00586-019-06201-z
Blizzard DJ, Nickel BT, Seyler TM, Bolognesi MP (2016) The impact of lumbar spine disease and deformity on total hip arthroplasty outcomes. Orthop Clin North Am 47(1):19–28. https://doi.org/10.1016/j.ocl.2015.08.005
Assi A et al (2023) ASD with high pelvic retroversion develop changes in their acetabular orientation during walking. Brain Spine 3:101752. https://doi.org/10.1016/j.bas.2023.101752
Klemt C, Padmanabha A, Tirumala V, Walker P, Smith EJ, Kwon Y-M (2021) Lumbar spine fusion before revision total hip arthroplasty is associated with increased dislocation rates. J Am Acad Orthop Surg 29(17):e860–e868. https://doi.org/10.5435/JAAOS-D-20-00824
Lum ZC, Giordani M, Meehan JP (2020) Total hip instability and the spinopelvic link. Curr Rev Musculoskelet Med 13(4):425–434. https://doi.org/10.1007/s12178-020-09648-6
Yang DS et al (2022) Risk of dislocation and revision following primary total hip arthroplasty in patients with prior lumbar fusion with spinopelvic fixation. J Arthroplasty. https://doi.org/10.1016/j.arth.2022.03.061
Di Martino A, Bordini B, Ancarani C, Viceconti M, Faldini C (2021) Does total hip arthroplasty have a higher risk of failure in patients who undergo lumbar spinal fusion? Bone Joint J 103-B(3):486–491. https://doi.org/10.1302/0301-620X.103B3.BJJ-2020-1209.R1
Wirries N, Schwarze M, Daentzer D, Skutek M (2020) Total hip arthroplasty and lumbar spine disorders: plain co-existence or mutual influence? Orthop Rev (Pavia). https://doi.org/10.4081/or.2020.8546
Eneqvist T, Nemes S, Brisby H, Fritzell P, Garellick G, Rolfson O (2017) Lumbar surgery prior to total hip arthroplasty is associated with worse patient-reported outcomes. Bone Joint J 99-B(6):759–765. https://doi.org/10.1302/0301-620X.99B6.BJJ-2016-0577.R2
Kozaki T et al (2022) “S2 alar-iliac screw loosening as a preventive factor for hip joint osteoarthritis after adult spinal deformity surgery: a case-control study. Eur Spine J 31(11):3081–3088. https://doi.org/10.1007/s00586-022-07344-2
Grotle M et al (2019) Lumbar spine surgery across 15 years: trends, complications and reoperations in a longitudinal observational study from Norway. BMJ Open 9(8):e028743. https://doi.org/10.1136/bmjopen-2018-028743
Mesregah MK et al (2022) Demographic, clinical, and operative risk factors associated with postoperative adjacent segment disease in patients undergoing lumbar spine fusions: a systematic review and meta-analysis. Spine J 22(6):1038–1069. https://doi.org/10.1016/j.spinee.2021.12.002
Onggo JR et al (2021) Comparable dislocation and revision rates for patients undergoing total hip arthroplasty with subsequent or prior lumbar spinal fusion: a meta-analysis and systematic review. Eur Spine J 30(1):63–70. https://doi.org/10.1007/s00586-020-06635-w
Malkani AL et al (2019) Does timing of primary total hip arthroplasty prior to or after lumbar spine fusion have an effect on dislocation and revision rates? J Arthroplasty 34(5):907–911. https://doi.org/10.1016/j.arth.2019.01.009
Mills ES et al (2022) Timing of total hip arthroplasty affects lumbar spinal fusion outcomes. Clin Spine Surg Spine Publ 35(2):E333–E338. https://doi.org/10.1097/BSD.0000000000001265
Buckland AJ, Abotsi EJ, Vasquez-Montes D, Ayres EW, Varlotta CG, Vigdorchik JM (2020) Lumbar spine degeneration and flatback deformity alter sitting-standing spinopelvic mechanics—implications for total hip arthroplasty. J Arthroplasty 35(4):1036–1041. https://doi.org/10.1016/j.arth.2019.11.020
Bala A et al (2019) Timing of lumbar spinal fusion affects total hip arthroplasty outcomes. JAAOS Glob Res Rev 3(11):e00133. https://doi.org/10.5435/JAAOSGlobal-D-19-00133
An VVG, Phan K, Sivakumar BS, Mobbs RJ, Bruce WJ (2018) Prior lumbar spinal fusion is associated with an increased risk of dislocation and revision in total hip arthroplasty: a meta-analysis. J Arthroplasty 33(1):297–300. https://doi.org/10.1016/j.arth.2017.08.040
Di Martino A et al (2021) Survival rates and reasons for revision of different stem designs in total hip arthroplasty for developmental dysplasia: a regional registry study. J Orthop Traumatol 22(1):29. https://doi.org/10.1186/s10195-021-00590-y
Malkani AL et al (2018) Total hip arthroplasty in patients with previous lumbar fusion surgery: Are there more dislocations and revisions? J Arthroplasty 33(4):1189–1193. https://doi.org/10.1016/j.arth.2017.10.041
Di Martino A et al (2021) Clinical and radiological outcomes of total hip arthroplasty in patients affected by Paget’s disease: a combined registry and single-institution retrospective observational study. J Orthopaed Traumatol 22(1):13. https://doi.org/10.1186/s10195-021-00574-y
Kikuchi S et al (2022) “Relationship between hip joint proximity area and sagittal balance parameters: an upright computed tomography study. Eur Spine J 31(2):215–224. https://doi.org/10.1007/s00586-020-06664-5
Ouchida J et al (2022) Impact of the hip joint mobility on whole-body sagittal alignment: prospective analysis in case with hip arthroplasty. Eur Spine J 31(9):2399–2407. https://doi.org/10.1007/s00586-022-07251-6
Pizones J, García-Rey E (2020) Pelvic motion the key to understanding spine–hip interaction. EFORT Open Rev 5(9):522–533. https://doi.org/10.1302/2058-5241.5.200032
Zagra L et al (2022) Current concepts in hip–spine relationships: making them practical for total hip arthroplasty. EFORT Open Rev 7(1):59–69. https://doi.org/10.1530/EOR-21-0082
Onggo JR et al (2021) “Comparable dislocation and revision rates for patients undergoing total hip arthroplasty with subsequent or prior lumbar spinal fusion: a meta-analysis and systematic review. Eur Spine J 30(1):63–70. https://doi.org/10.1007/s00586-020-06635-w
Windsor EN, Sculco PK, Mayman DJ, Vigdorchik JM, Jerabek SA (2022) Spinopelvic hypermobility corrects after staged bilateral total hip arthroplasty. HSS J 18(4):541–549. https://doi.org/10.1177/15563316211050353
Langer S, Stephan M, von Eisenhart-Rothe R (2021) Importance of hip-spine syndrome in hip arthroplasty: influence on the outcome and therapeutic consequences”. Z Orthop Unfall. https://doi.org/10.1055/a-1527-7697
Funding
Open access funding provided by Alma Mater Studiorum - Università di Bologna within the CRUI-CARE Agreement. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by BB, GG, CA, CD’, MB, CG, MV, and ADM. The first draft of the manuscript was written by Cesare Faldini, and all authors commented on previous versions of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Di Martino, A., Bordini, B., Geraci, G. et al. Impact of previous lumbar spine surgery on total hip arthroplasty and vice versa: How long should we be concerned about mechanical failure?. Eur Spine J 32, 2949–2958 (2023). https://doi.org/10.1007/s00586-023-07866-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-023-07866-3