Abstract
Purpose
We evaluated the long-term safety, mobility and complications of cervical total disc arthroplasty with the Baguera®C prosthesis over 10 years.
Methods
We included 91 patients treated by arthroplasty for cervical degenerative disc disease. A total of 113 prostheses were implanted (50 one-level, 44 two-level and 19 hybrid constructs). They were assessed for complications, clinically, with NDI and SF-12 questionnaires and by independent radiologists for ROM, HO, disc height and adjacent level degeneration.
Results
No spontaneous migration, loss of fixation, subsidence, vascular complication or dislocation were observed. The reoperation rate was 1%. About 82.7% of the patients were pain free. About 9.9% were taking occasional grade I painkillers. Motricity and sensitivity were preserved in 98.8% and 96.3%. The NDI showed an average functional disability of 17.58%, 26% lower than preoperatively. The SF-12 scores were close to normal health. The average ROM at the treated level was 7.4°. Motion was preserved in 86.6%. Lack of motion was observed in 13.4%. Grades II and III H0 were present in 53.7% and 31.7%, respectively, Grade IV was present in 13.4%. Motion was preserved in 100% of the grades 0–III. The preoperative adjacent level disc height of 4.3 mm remained stable during all the follow-ups at 4.4 mm and 4.2 mm, respectively, at 5 and 10 years.
Conclusions
After 10 years, cervical arthroplasty with the Baguera®C prosthesis presents excellent safety and functional results and low complications. Motion was preserved in 86.6%, with a 7.4° ROM. Although common, HO did not hinder motion. Adjacent disc height preservation confirms some adjacent level degeneration protection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cervical arthroplasty has been widely used over the past two decades. The selection criteria for adequate surgical indications have progressively improved along with the design of the implants to make arthroplasty a validated tool for the treatment of degenerative disc lesions in many countries [1,2,3]. Still, very long follow-up results may be missing particularly for the latest designed implants such as semi-constrained prostheses.
The Baguera®C (Spineart Inc., Switzerland) is a semi-constrained cervical disc prosthesis available on the EU market since 2007. The implants include two plates and a mobile nucleus, thus allowing for a mobility of 8° of arc, in all directions, while assuring stability thanks to an anatomical shape and six fins at the rear side of the plates. The device is made of a titanium alloy, DLC (diamond-like carbon) and a high-density biocompatible polymer for the core.
Its major differences with other implants are the unique association of a three-level stabilization system (three metal fins implanted in the endplate, anatomical shape and titanium plasmapore coating allowing osteointegration), a diamond-like carbon coating interface between the polyethylene nucleus and the titanium endplates (to reduce friction, wear debris and increase the implant longevity) and a guided mobile nucleus, clipped in the implant and designed to prevent excessive constraints to the facet joints and to avoid posterior subluxation of the nucleus. The diamond-like coating and the titanium also produce minimal artefacts under MRI for a better postoperative follow-up. Finally, the shape of the PE nucleus is designed for shock absorption in order to limit stress transfer to adjacent levels (Fig. 1).
The Baguera C cervical disc prosthesis is composed of two anatomical diamond-like coated ISO 5832-3 titanium endplates and a UHMWPE ISO 5834-2 shock absorbing polyethylene nucleus. Primary stability is provided by the anatomical shape and by three fins on each endplate whereas secondary stability is obtained by surface osteointegration
We evaluated the long-term safety, potential late complications and long-term performance related to the use of the Baguera®C prosthesis, in an observational multicentric study over a 10 years postoperative follow-up.
Material and methods
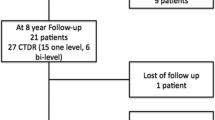
This prospective observational study is an extension of a 2-year follow-up registry on 118 patients who were treated at one or more levels in five European centres between June 2009 and June 2011. After withdrawal of one investigational centre, 91 patients were enrolled in this study to return for a follow-up visit at 5 years and 10 years post-surgery (Fransen 2017, Fransen 2018). The study aims at evaluating the long-term safety, mobility-related benefits and potential late complications related to the use of the prosthesis.
90/91 patients completed the 5-year follow-up visit. At 10 years, 81/91 patients could be assessed for clinical, radiological and safety data. Two patients died at 9.5- and 10.9-year post-surgery of causes unrelated to the surgery or device, and eight were lost to follow-up between the 5- and 10-year visits.
The age at surgery time was 44.2 ± 8.5 years. The distribution by gender was 45 females (49.5.5%) and 46 males (50.5%). The BMI (kg/m2) at surgery time was 25.9 ± 4.9 concerning 89 patients.
The indication for surgery was symptomatic cervical degenerative disease presenting with neck or arm pain. All patients had arm pain, four patients had arm pain only and no patients were treated for neck pain only. On purpose, no patients with myelopathy were enrolled in this study.
A total of 113 prostheses were implanted. Fifty patients had one-level disc replacement, 44 had two levels and 19 had hybrid constructs (prosthesis + cage). The most frequently implanted level was C5–C6 (53 levels—47%) followed by C6–C7 (40 levels—35.4%). Seventeen patients (15%) had surgery at C4–C5 and three patients at C3–C4 (2.6%).
Safety was assessed by the rate of the following complications: surgical revision at the treated level, explantation (removal) of the prosthesis, fracture of system components, loss of fixation, migration, fracture of a vertebra and neurological or vascular disorders.
Performance was assessed by clinical and neurological examination, functional evaluation using the Neck Disability Index (NDI) self-reported questionnaire and quality of life using the SF-12 self-reported questionnaire.
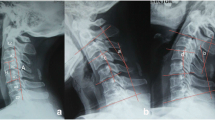
Radiological mobility was assessed by range of motion angle (ROM angle) at the treated level, measured at a radiological check-up, using Rx images taken in flexion and extension. The radiological assessments were performed centrally by an independent image assessment laboratory, using a semi-automatic and validated process with verification by an independent radiologist [4].
Heterotopic ossification (HO) was assessed from the medical pictures and based on the modified McAfee and Mehren classification [5, 6].
Subsidence was not defined nor measured.
The Case Report File was completed at each patient control visit, safety data were collected continuously, during the overall study period, using adverse events forms. Complications were reported in specific forms. On-site monitoring was performed on the source data for all patients.
Results
During all the 10 years follow-up, no spontaneous migration, loss of fixation, subsidence and fracture of system component were discovered. One patient presented neck pain following a fall and neck injury 7 years after surgery, causing possible implant loosening. The device was removed, and the patient underwent fusion. This adverse event was reported as unrelated to the device or surgery. There was no reoperation for adjacent level degeneration. Four surgeries were performed at another spine level, more specifically at lumbar levels. No vascular injury, serious neurological complications or vertebral fractures were reported.
The pain medication was globally assessed after 10-year follow-up (FU) for each patient, without details to check for association with the cervical arthroplasty. The pain medication has been categorized as non-narcotic medication = Grade 1/weak narcotic = Grade 2/strong narcotic medication = Grade 3 analgesic. No medication was taken by 82.7% of the patients. Grade I painkillers were observed in 9.9%, Grade 2 in 6.2 and Grade 3 in 0%. Two patients (2.5%) were following alternative pain therapies such as muscle relaxants.
The motor function was normal at 10 years for 80 of the 81 patients (98.8%) while one patient showed decreased active movement, when tested against some resistance. At 10 years, all the patients showed normal reflexes (100%). Finally, 77 of the 80 observed patients (96.3%) showed normal sensory examinations while three patients showed impaired light touch.
The NDI self-reported questionnaires showed an average functional disability of 17.58% ± 16.34% at 10 years (n = 80)—one-level TDR (43 patients): 15.95% ± 16.87%—two-level TDR (20 patients): 18.40% ± 16.41%—hybrid surgeries (17 patients): 20.71% ± 15.26%. NDI remained stable between the 5- and 10-year FU visits. At 10 years, the overall mean NDI is 26% lower than the pre-surgery value of 44.13 (± 15.88).
The SF-12 scores were calculated at 10 years for 81 patients using the Quality Metric SF-12V2 software. The Physical Component Summary PCS-12 values at 10 years reached 45.19%, and the Mental Component Summary MCS-12 values at 10 years reached 42.34%, and the Vitality Scores VT reached 40.36%, all close to normal health.
The average ROM at the treated level at 10 years (n = 82) was 7.4° ± 4.3°. Motion was considered preserved (ROM ≥ 2°) in 71 levels (86.6%). Lack of motion (ROM < 2°) was observed in 11 levels (13.4%).
The ROM evolution over time including all patients for whom a preoperative ROM value and at least one ROM value at 5 or 10 years is available, shows preserved motion in over 83% of the patients at all times. The ROM decreased between 2 years (93.2%) and 5 years (82.8%) post-surgery, but was stable between the 5y FU and the 10y FU (83%) (Figs. 2 and 3).
The ROM was also evaluated according to the patient’s age, above or under 50 years at the time of surgery. Overall, the ROM was preserved in 83.7% of the patients under 50 years old and 93.8% over 50 years. For one-level arthroplasty, ROM was preserved in 84.2% < 50 and 80% > 50, whereas for two-level arthroplasty, the ratio was 81.3% preserved ROM < 50 and 100% preserved ROM > 50. In hybrid cases, preserved ROM was 87.5% < 50 and 100% > 50.
Heterotopic ossification was assessed at 10 years for 82 operated levels. No patient presented grade 0 HO, and one patient presented grade I (1.2%). Grade II (HO present in the intervertebral disc space with possible affection of the function of the prosthesis) was seen in 44 patients (53.7%), grade III (bridging ossification which still allows movement of the prosthesis) in 26 patients (31.7%) and grade IV (complete fusion) in 11 patients (13.4%). The link between ROM and HO classification at 10 years FU showed preserved motion in 100% of the grades 0–3 and lack of motion in 100% of grade 4 patients.
Finally, an analysis of the adjacent disc mean height (upper level) was performed at 5 years and 10 years to assess possible progressive degeneration. The preoperative mean disc height at the upper adjacent level was 4.6 mm (± 0.9). It remained stable during all the follow-up: 4.4 (± 1.1) mm and 4.2 (± 1.3) mm, respectively, at 5 years (n = 67) and 10 years (n = 62).
Discussion
Despite the absence of control group, a relatively small number of patients and the presence of hybrid cases that may behave differently, this prospective long-term study offers some valuable information.
Clinical and radiologic evaluations at 5 and 10 years postoperative controls of patients treated for symptomatic cervical degenerative disease, by arthroplasty at one or two levels using the Baguera®C, cervical prosthesis, confirm that both the device and procedure are safe, with a low rate of complications and with good clinical and functional outcomes. We did not observe direct implant failure or complications such as vertebral fracture, dislocation or migration, therefore not confirming the 4.7% failure rate reported by Zafras et al. in their 2022 metanalysis [7]. As subsidence was not measured, no conclusions could be drawn about its occurrence.
Joo et al. reported a 5-year reoperation rate with ACDF between 13 and 22% depending on the number of treated levels [8]. A low reoperation rate for arthroplasty confirms the previous studies that were focusing on the comparison between arthroplasty and ACDF [9,10,11,12]. We only observed one reoperation after a direct neck trauma 7 years after the initial disc replacement.
Reoperation rates in cervical arthroplasty for 10-year FU studies have been reported previously, ranging from 2 to 10.3%, depending on the study group and on the implant [10, 11, 13,14,15,16,17] (Table 1). This low reoperation rate could be explained by each participating surgeon’s indications for surgery, by the surgical technique that may differ from one implant to the other, but Lee et al. also suggested the importance of the implant design mimicking the natural centre of rotation during flexion and allowing wider distribution and lower contact pressure on the core [18].
Angular motion and motion preservation have also been reported in various studies (Table 2). Our results confirm the long-term preservation of a significant range of motion, between 6.1° and 10.2° depending on the implant type [10, 13, 16, 17, 19,20,21,22,23].
Adjacent level degeneration can be assessed by operation at a level adjacent to the operated level or by progressive loss of height of the adjacent level. In this study, no patient had to be operated at another level of the cervical spine during the whole length of the FU. Also, the adjacent level disc height showed only a minimal decrease over the 10 years period, compatible with normal ageing. These results compare favourably to the rate of adjacent level surgery in other published long-term FU series of arthroplasty cases [13, 14, 16, 17, 21, 23] (Table 3).
No progressive osteolysis was observed. Noteworthy, there was no progression of the reported asymptomatic blunting of the anterior corner of the vertebral bone seen in the same group of patients during the 5 years FU study [25]. This could be caused by early bone remodelling after the implantation of the prosthesis, stable afterwards, but should be confirmed by specific studies.
Finally, we observed a slightly better range of motion for the patients aged over 50 at the time of surgery, in two-level surgeries, whether double arthroplasties—93%—or hybrid cases—100%—associating arthroplasty and fusion. Although this was not observed in the one-level arthroplasties where the ROM was slightly lower over 50 (80% vs 84%), this could mean that age as such may not be a definitive selection criterion, and that indications for arthroplasty should be influenced more specifically by disc height, facet arthritis and spinal canal diameter.
Conclusion
Confirming previous studies [24, 25], this 10-year follow-up series of cervical disc replacement with the Baguera®C prosthesis showed excellent safety and functional results and low complications. The index level reoperation rate was 1%. Motion was preserved in 86.6% of the patients, with a mean 7.4° ROM. About 92.6% of the patients were either pain free or occasionally taking level I painkillers. Although grades II and III heterotopic ossifications were common, they did not hinder motion. Adjacent disc height was preserved compared to the preoperative findings, confirming better adjacent level degeneration protection than what has been reported with long-term ACDF [26]. Finally, in this study, age over 50 did not corelate with worse radiological results.
References
Depreitere B, Fransen P, Goffin J, Lubansu A, Put E, Scordidis V, Van Schaeybroeck P (2008) Recommendations of good practice for cervical disc replacement. In: Presented at the Société Belge de Neurochirurgie, Liège
Fransen P, Pointillart V (2016) Arthroplasty with the Baguera®C cervical disc prosthesis: review of the scientific background, clinical and radiographic evidences. J Spine Neurosurg 5:6
Joaquim A, Riew K (2017) Multilevel cervical arthroplasty: current evidence. A systematic review. Neurosurg Focus 42(2):E4
Fransen P, Hansen-Algenstaedt N, Chatzisotiriou A, Gonzalez Noriega D, Verheyden J, VanHecke W, Pointillart V (2016) Radiographic outcome and adjacent segment evaluation two years after cervical disc replacement with the Baguera®C prosthesis as treatment of degenerative cervical disc disease. J Spine 5:2
McAfee PC, Cunningham BW, Devine J, Williams E, Yu-Yahiro J (2003) Classification of heterotopic ossification (HO) in artificial disk replacement. J Spinal Disord Tech 16(4):384–389
Mehren C, Suchomel P, Grochulla F, Barsa P, Sourkova P, Hradil J, Korge A, Mayer HM (2006) Heterotopic ossification in total cervical artificial disc replacement. Spine 31(24):2802–2806
Zafras A, Sullivan T, Singh K, Phillips F, Colman M (2022) Failure an-in cervical total disc arthroplasty: single institution experience, systematic review of the literature, and proposal of the RUSH TDA failure classification system. Spine J 22:353–369
Joo P, Zhu J, Kammien A, Gouzoulis M, Arnold P, Grauer J (2022) Clinical outcomes following one-, two-, three- and four-level anterior cervical discectomy and fusion: a national database study. Spine J 22:542–548
Coric D, Nunley PD, Guyer RD, Musante D, Carmody CN, Gordon R, Lauryssen C, Ohnmeiss DD, Boltes MO (2011) Prospective, randomized, multicenter study of cervical arthroplasty: 269 patients from the Kineflex|C artificial disc investigational device exemption study with a minimum 2-year follow-up: clinical article. J Neurosurg Spine 15(4):348–358
Radcliff K, Davis R, Hisey M, Nunley P, Hoffman G, Jackson R, Bae H, Albert T, Coric D (2017) Long term evaluation of cervical disc arthroplasty with the Mobi-C cervical disc: a randomized, prospective, multicenter clinical trial with seven years follow-up. Int J Spine Surg 11(4):31
Vaccaro A, Beutler W, Peppelman W, Marzluff J, Mugglin A, Ramakrishnan P, Myer J, Baker K (2018) Long term clinical experience with selectively constrained Secure-C cervical artificial disc for 1-level cervical disc disease: results from seven-year follow-up of a prospective, randomized, controlled investigational device exemption clinical trial. Int J Spine Surg 12(3):377–387
Vaccaro A, Beutler W, Peppelman W, Marzluff JM, Highsmith J, Mugglin A, DeMuth G, Gudipally M, Baker KJ (2013) Clinical outcomes with selectively constrained SECURE-C cervical disc arthroplasty: two-year results from a prospective, randomized, controlled, multicenter investigational device exemption study. Spine 38(26):2227–2239
Dejaegher J, Walraevens J, van Loon J, Van Calenbergh F, Demaerel P, Goffin J (2017) 10-year follow-up after implantation of the Bryan cervical disc prosthesis. Eur Spine J 26(4):1191–1198
Gornet MF, Burkus JK, Shaffrey ME, Schranck FW, Copay AG (2019) Cervical disc arthroplasty: 10-year outcomes of the Prestige LP cervical disc at a single level. J Neurosurg Spine 31(3):317–325
Gornet MF, Lanman TH, Burkus JK, Dryer RF, McConnell JR, Hodges SD, Schranck FW (2019) Two-level cervical disc arthroplasty versus anterior cervical discectomy and fusion: 10-year outcomes of a prospective, randomized investigational device exemption clinical trial. J Neurosurg Spine 31:508–518
Kim K, Hoffman G, Bae H, Redmond A, Hisey M, Nunley P, Jackson R, Tahernia D, Araghi A (2021) Ten-year outcomes of 1- and 2-level cervical disc arthroplasty from the Mobi-C investigational device exemption clinical trial. Neurosurgery. 88(3):497–505
Mehren C, Heider F, Siepe CJ, Zillner B, Kothe R, Korge A, Mayer HM (2017) Clinical and radiological outcome at 10 years of follow-up after total cervical disc replacement. Eur Spine J 26(9):2441–2449
Lee J, Park W, Kim Y, Jahng T (2016) A biomechanical analysis of an artificial disc with a shock-absorbing core property by using whole-cervical spine finite element analysis. Spine 41(15):893–901
Song Q, He D, Han X, Zhang N, Wang J, Tian W (2018) Clinical and radiological outcomes of cervical disc arthroplasty: ten-year follow-up study. Int Orthop 42(10):2389–2396
Pointillart V, Castelain JE, Coudert P, Cawley DT, Gille O, Vital JM (2018) Outcomes of the Bryan cervical disc replacement: fifteen year follow-up. Int Orthop 42(4):851–857
Lavelle WF, Riew KD, Levi AD, Florman JE (2019) Ten-year outcomes of cervical disc replacement with the BRYAN cervical disc: results from a prospective, randomized, controlled clinical trial. Spine 44(9):601–608
Genitiempo M, Perna A, Santagada D, Meluzio M, Projetti L, Bocchi M, Logroscino C, Tamburelli F (2020) Single-level Bryan cervical disc arthroplasty: evaluation of radiological and clinical outcomes after 18 years of follow-up. Eur Spine J 29(11):2823–2830
Zhao Y, Zhou F, Sun Y, Pan S (2020) Single-level cervical arthroplasty with ProDisc-C artificial disc: 10-year follow-up results in one centre. Eur Spine J 29(11):2670–2674
Fransen P, Hansen-Algenstaedt N, Chatzisotiriou A, Gonzalez Noriega D, Pointillart V (2018) Clinical results of cervical disc replacement with the Baguera C prosthesis after two years follow-up. Acta Orthopedica Belgica 84(3):345–351
Fransen P, Noriega D, Chatzisotiriou A, Pointillart V (2018) One or two levels treatment by arthroplasty of cervical degenerative disease. Preliminary results after 5 years postoperative controls. J Spine 7:1
Wang F, Hou HT, Wang P, Zhang JT, Shen Y (2017) Symptomatic adjacent segment disease after single-lever anterior cervical discectomy and fusion: incidence and risk factors. Medicine (Baltimore) 96(47):e8663
Acknowledgements
The monitoring mission of the study was supported by a CRA appointed by the study's sponsor, Spineart, for the duration of the study. The radiological assessment was performed by an independent image analysis laboratory, IcoMetrix NV, Leuven, Belgium. Spineart contracted with participating institutions/investigators through a Clinical Trial Agreement (CTA) that defines the scope and responsibilities and associated compensation related to carrying out the obligations under a clinical study sponsored by Spineart. The CTA has been retained in the Trial Master File.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
This study has been conducted according to the ISO 14155:2011 standard and any national regulations applicable in the participating countries. Investigators worked in compliance with Good Clinical Practices defined by the competent authorities and according to all existing regulations at local, national and European level. This study was approved by the Ethics Committees of the involved medical institutions. The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
This study is conducted outside the US and is not intended for submission to the FDA. In Europe, Baguera C is CR marked since 2007 and is MDR CE mark certified since 2022. In the US, Baguera C is investigational/not approved for use. Two IDE are in process, i.e. a RCT comparing Baguera C with Mobi-C at one-level and two-level total disc replacement.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fransen, P., Noriega, D., Chatzisotiriou, A. et al. Cervical disc arthroplasty with the Baguera C prosthesis: clinical and radiological results of a 10-year follow-up study. Eur Spine J 32, 3533–3539 (2023). https://doi.org/10.1007/s00586-023-07833-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-023-07833-y