Abstract
Purpose
To provide an overview of the The Norwegian Degenerative spondylolisthesis and spinal stenosis (NORDSTEN)-study and the organizational structure, and to evaluate the study population.
Methods
The NORDSTEN is a multicentre study with 10 year follow-up, conducted at 18 public hospitals. NORDSTEN includes three studies: (1) The randomized spinal stenosis trial comparing the impact of three different decompression techniques; (2) the randomized degenerative spondylolisthesis trial investigating whether decompression surgery alone is as good as decompression with instrumented fusion; (3) the observational cohort tracking the natural course of LSS in patients without planned surgical treatment. A range of clinical and radiological data are collected at defined time points. To administer, guide, monitor and assist the surgical units and the researchers involved, the NORDSTEN national project organization was established.
Corresponding clinical data from the Norwegian Registry for Spine Surgery (NORspine) were used to assess if the randomized NORDSTEN-population at baseline was representative for LSS patients treated in routine surgical practice.
Results
A total of 988 LSS patients with or without spondylolistheses were included from 2014 to 2018. The clinical trials did not find any difference in the efficacy of the surgical methods evaluated. The NORDSTEN patients were similar to those being consecutively operated at the same hospitals and reported to the NORspine during the same time period.
Conclusion
The NORDSTEN study provides opportunity to investigate clinical course of LSS with or without surgical interventions. The NORDSTEN-study population were similar to LSS patients treated in routine surgical practice, supporting the external validity of previously published results.
Trial registration
ClinicalTrials.gov; NCT02007083 10/12/2013, NCT02051374 31/01/2014 and NCT03562936 20/06/2018.
Similar content being viewed by others
Background
Lumbar spinal stenosis (LSS) is the most common indication for spine surgery in the age group above 65, and the rates of LSS surgery are increasing [1,2,3]. Patients with mild to moderate symptoms may have satisfactory long-term outcomes without surgical treatment [4, 5], but prospective long-term observational studies are scarce.
The main objective of the surgical treatment for LSS is to decompress neural structures to alleviate pain and improve function. There are several surgical decompression techniques available, but evidence for recommending one technique over the other is limited [6]. In the USA and Australia, the use of complex fusion has increased greatly [3, 7], even if these procedures are more costly and may put patients at risk for more serious complications [3, 7, 8]. Adding fusion surgery to the decompression in cases with LSS and degenerative spondylolisthesis (DS), is controversial issue in spine surgery.
Hence, The Norwegian Degenerative spondylolisthesis and spinal stenosis (NORDSTEN) study, a large nationwide multicentre study with a 10 year follow-up, was initiated to generate more evidence to improve management of LSS. NORDSTEN consists of the following main studies:
A. In the spinal stenosis trial (NORDSTEN-SST) patients were randomized to three different decompression techniques: spinous process osteotomy, bilateral laminotomy and unilateral laminotomy with crossover [9].
B. In the degenerative spondylolisthesis trial (NORDSTEN-DS) patients with LSS and concurrent degenerative spondylolisthesis were randomized to surgical decompression alone (DA) or decompression with instrumental fusion (DF) [10].
C. In the observational cohort (NORDSTEN-OC) we followed the patients with radiographic and symptomatic LSS with and without DS, who had symptom burden judged not severe enough to opt for surgical treatment.
Crucial for the external validity is that patients included are representative of those not included in this study, i.e., those receiving the standard package of care, according to surgeons and patients’ preferences. Information on these patients is recorded in the Norwegian Registry for Spine Surgery (NORspine).
The main purpose of the present paper is to give an overview of the NORDSTEN study, to present the study organization and to evaluate if the surgically treated NORDSTEN-study population is similar to patients reported to the National Registry for Spine Surgery (NORspine).
Methods
Study governance and organization
In order to govern, guide and follow-up all aspect of the NORDSTEN study, a national project organization was established (Fig. 1).
The Norwegian Degenerative spondylolisthesis and spinal stenosis (NORDSTEN) organization. 1. Members: head of the NORDSTEN study, head of scientific board, investigators for each study, head of NORDSTEN coordinating center and head of finances. 2. Members: all Administrative Executive Board members, principal investigators (PIs) from all four health regions in Norway, an international researcher and a patient representative. 3. An independent data monitoring committee at Clinical Trial Unit (CTU) at Oslo University Hospital (OUH). 4. Members: local PIs (surgeons) and study coordinator from each of the recruiting hospitals, and staff from the national NORDSTEN coordinating center. Responsibilities: recruit, treat and follow-up of patients in accordance with the standard procedures and guidelines developed by the Scientific Board and the Administrative Executive Board. Also, an external study monitor supervising activities according to Good Clinical Practice. 5. The Research and Communication Unit for Musculoskeletal Health (FORMI) at OUH is responsible for the nationwide coordination of the NORDSTEN study. Responsible for daily administration and support to the local coordinators, the randomization process and data management (collection and recording). Reports to the independent data monitoring committee at CTU/OUH
Biannual meetings/conferences with the scientific board, administrative executive board, working group, patient representative, study monitor and the study coordination center were arranged to ensure adherence to the study protocol and to inform about the progress of the study.
Study population
Patients with symptomatic LSS referred to orthopedic or neurosurgical outpatient clinics at public hospitals were eligible to the study. Inclusion and exclusion criteria are listed in Table 1, and detailed in protocol articles [9, 10].
Patients were offered participation in one of the three studies (Fig. 2).
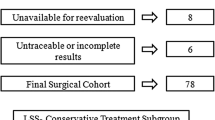
Participation of patients in the NORDSTEN study. Flow chart of The Norwegian Degenerative spondylolisthesis and spinal stenosis (NORDSTEN) study displaying the four potential outcomes after screening patient at the outpatient clinic at 18 Norwegian public hospitals: 1) patient did not fill inclusion criteria or filled exclusion criteria—excluded, 2) patient not opted for surgical treatment—observation cohort (OC), 3) patient had indication for surgery (without spondylolisthesis)—the spinal stenosis trial (SST), and 4) patient had indication for surgery (with spondylolisthesis)—the degenerative spondylolisthesis trial (DS)
Inclusion started in February 2014 and ended in October 2018, and baseline data for the current analysis were available when 2 year follow-up was completed.
Study interventions and randomization
Shared decision-making between surgeon and patient determined if surgical or nonsurgical treatment would be implemented. If opted for surgery, patients were eligible for the randomized trials, if not; they were eligible for the OC. Surgical interventions used in NORDSTEN are documented in detail in protocol articles [9, 10] and shown in short in Table 2.
All participating surgeons were experienced with the treatments used in the trials. Prior the start of the trials, principal investigators (PIs) from the Scientific Board visited the hospitals to ensure a common understanding and performance of the surgical methods described in study protocols.
Randomization in the two interventional trials was carried out within a 6 week period prior to surgery. Randomization lists were computer generated, center-stratified and block permuted with a 1:1:1 (SST trial) or 1:1 (DS trial) allocation and performed within the Medinsight database (version 2.17.9), a research database developed and owned by Oslo University Hospital.
Data collection and monitoring
Data are/were collected preoperatively (baseline), and at 3 months, 1, 2, 5 and 10 year postoperatively (Fig. 3). Data are/were collected in collaboration between local coordinators and NORDSTEN-study coordinating center (FORMI).
FORMI, the Clinical Trials Unit (CTU) at Oslo University Hospital and the external study monitor are responsible for data safety and quality. The data control plan included automatic and manual checks of data quality at defined time intervals. Plotting of primary outcome was verified for all research subjects at all follow-up intervals, all other data entered were verified for every fifth research participant number (20%). In addition, an agreement outlined a database lock until 2 year follow-up was completed, and how data were made available.
As an intervention study it was decided to implement the Good Clinical Practice (GCP) guidelines in the NORDSTEN-SST and -DS trials in order to add quality, increase resource efficiency and safety [12]. The external study monitor visited study sites regularly throughout the first 2 year follow-up (Table 3), and reviewed all included patients regarding deviations from the protocol.
All scientific board members, PIs, local study coordinators and staff at the NORDSTEN-study coordinating center underwent GCP certification course prior to study commencement.
Study variables
The present paper presents baseline data collected both in the NORDSTEN study and in the NORspine registry: 1) descriptive baseline characteristics (age, gender, level of education, work status, smoking habits, marital status, duration of back pain history, former back surgery and American Society of Anesthesiologists classification (ASA); 2) the Norwegian validated version of Oswestry Disability Index (ODI) version 2.0 [13] as the primary outcome measure; and 3) Numeric Rating Scale (NRS) for back and leg pain, EuroQol 5 dimensions questionnaire (EQ-5D) as secondary outcome measures.
In the NORDSTEN study some variables specific for patients with LSS were added: the Norwegian validated version of the Zürich Claudication Questionnaire (ZCQ) and the Hopkins symptom check list (emotional distress) [9, 10]. A range of radiological measurements were performed in all NORDSTEN studies. Case report forms (CRF’s) for registration of adverse events were designed [12]. All data were collected by paper. At hospital admission, patients completed questionnaires, which included patient reported outcome measures (PROMs) and questions about demographics and lifestyle. The surgeons recorded surgical parameters.
The local coordinators (not involved in the treatment) reported complications/adverse events (including reoperations) in CRFs. The external monitor cross-checked all reported data in the CRF’s against clinical patient journal. In the present paper, monitoring report is only reported preoperatively.
Radiological evaluations in NORDSTEN: standing X-ray with functional images and standard MRI of the lumbar spine, including T1 and T2 sequences in the axial and sagittal planes were performed on all patients at baseline. See Fig. 3 for radiological follow-ups.
Study design and statistical analysis
Main endpoint for the NORDSTEN-SST and NORDSTEN-DS is at 2 year follow-up, and for NORDSTEN-OC at 5 year follow-up.
The SST trial is a superiority trial comparing three surgical decompression techniques [9]. The DS trial is a non-inferiority trial comparing DA and DF [10]. The reporting of the two randomized trials follows CONSORT (Consolidated Standards of Reporting Trials) checklists for reporting randomized trials. For the OC study, STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) guidelines will be used. A statistician, blinded to treatment allocation, conducted the statistical evaluations of main outcomes in the two randomized NORDSTEN trials. Statistical analysis plans were published before data were made accessible.
Representativeness of the NORDSTEN-study population
To evaluate if the patients enrolled in the NORDSTEN study were similar to patients operated in an ordinary clinical setting in Norway, baseline data from the NORspine, a national quality registry for surgical treatment for degenerative disorders in the cervical and lumbar spine, were used. Preoperative data registered in NORspine from the same hospitals on corresponding patient groups treated consecutively in the same period were used to describe baseline characteristic of those not included in NORDSTEN study.
Descriptive comparison between patients included in the two NORDSTEN randomized trials and patients reported in the NORspine registry was done without direct statistical comparison as the intention was to judge if the patient populations were similar.
Results
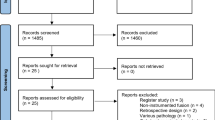
The 18 recruiting hospitals (19 study sites) were both university hospitals and smaller public hospitals located in all regions of Norway (Table 3). In total 2227 patients were screened for eligibility at outpatient clinics between February 2014 and September 2018 (Fig. 4). Seven hundred and four (32%) were included and operated for LSS with or without spondylolistheses in the randomized NORDSTEN trials (267 + 437), whereas 284 patients were included in the observation cohort. Mean time from randomization to surgery was 12.4 (SD 21.9) and 16.9 (SD 22.4) days in the SST- and DS trial, respectively. The number of patients reported to the NORspine registry from the same hospitals, the same time period and with the same inclusion/exclusion criteria as in the NORDSTEN study was 2908 of 4310 (67%). This means that about 1 of 5 of available patients (704/3612) from the participating hospitals were included in the randomized NORDSTEN trials.
Patient selection in in the NORspine registry and the NORDSTEN study. Flow chart of the Norwegian Registry for Spine Surgery (NORspine) and The Norwegian Degenerative spondylolisthesis and spinal stenosis (NORDSTEN) study. Patients from the NORspine were selected by the same inclusion and exclusion criteria as in NORDSTEN
Baseline characteristics of the NORDSTEN and the NORspine cohorts are shown in Table 4. Only minor differences were registered between patients included in the randomized NORDSTEN trials and other patients operated at the 18 participating hospitals and reported to the NORspine registry in the same period.
Monitoring the NORDSTEN study detected deviations (Table 5). Most of them were minor (i.e., radiology and PROMs completed out of set time period, informed consent not signed correctly).
Brief results from NORDSTEN papers 2 years postoperatively
NORDSTEN-SST reported no differences in clinical outcomes or complication rates among the 3 minimally invasive posterior decompression techniques used to treat patients with lumbar spinal stenosis [15]. NORDSTEN-DS found that in patients operated for degenerative lumbar spondylolisthesis decompression was noninferior to decompression with instrumented fusion [14].
Discussion
The NORDSTEN study is an ongoing multicentre study including 988 patients suffering from LSS with or without degenerative spondylolisthesis recruited over a period of 4.5 years. The aim of NORDSTEN study is primarily to evaluate the efficacy of different surgical methods for treating patients with LSS. The comprehensive organization of the NORDSTEN study is considered to ensure high quality and control in the planning process, patient recruitment and treatment and follow-up phases.
Planning
Large resources were used in the planning phase of the NORDSTEN study to ensure open discussions carried out in the formation of the research protocols [9, 10]. The decision to prospectively follow disease progress of included patients by PROMs and radiological imaging during the 10 years of follow-up creates a unique opportunity to improve knowledge and routines related to clinical practice, and increased precision implementation of imaging and surgical procedures.
It is of outmost importance to ensure that the patients enrolled in the randomized trials are representative to patients treated in routine surgical practice. The inclusion and exclusion criteria were set to allow for generalization to the majority of patients with LSS evaluated for surgical treatment. External validity was therefore controlled by comparison of the NORspine registry once the database lock was suspended in adherence with the data security plan (completion of 2 year follow-up for SST and DS trials).
Strengths and limitations
Standardization of surgical procedures is challenging, but measures were taken to harmonize the execution of the surgical interventions at the eighteen hospitals. However, the design of the NORDSTEN study was also pragmatic in giving surgeons choice regarding surgical methods and instrumentation [9, 10]. The NORDSTEN randomized trials were not planned with control groups (e.g., sham surgery) and were not designed to evaluate the placebo effect. Neither were the patients blinded for treatment allocation.
As part of the shared decision-making regarding treatment, patients should be informed about the present evidence of the efficacy of surgery. Inclusion of patients in the NORDSTEN trials was conducted by spine surgeons. According to a Cochrane report, high quality research is needed in order to conclude about the benefits of surgical versus nonsurgical treatment [16]. Therefore, it is a limitation that the NORDSTEN did not include nonsurgical treatment as an arm in the study. The observation cohort in NORDSTEN is a selective cohort and cannot be applied to evaluate the natural course of LSS in general; however, it can tell the story of patients with LSS referred for surgery not operated.
The importance of the patient’s perspective in the evaluation of treatment has been generally recognized, and several types of patient-based outcome measures have been developed. The use of PROMs translated and validated for the Norwegian population and recommended by international panels of experts [17] ensured valid results and conclusions. The ODI was chosen as the primary outcome because it is the most commonly used back-specific measure that has been found reliable and valid despite that ODI was primarily designed to evaluate back pain. The less frequently used Zurich Claudication Questionnaire (ZCQ) was added due to the instruments specificity regarding the evaluation of function for LSS populations [18]. Standardized and well documented methods were used for data collection and analysis, and all main analyses are performed by statisticians blinded for treatment allocation.
The study organization, responsibilities and tasks were carefully planned in the early phase to ensure a good completion of the study. We have experienced that our research network has been robust with dedicated people at all levels which has been decisive for the results.
Standardized routines for study hospitals, study personnel and study coordinating center along with close collaboration between all trials organizations could be a contributing factor to the low dropout rates. In addition, informational letters regarding study progression and layman summaries have been sent out to study patients in collaboration with the study’s patient representative.
The uneven recruitment of patients to the SST and DS studies versus the OC study, is primarily due to the organization and routines at the different hospitals. At some university hospitals, many of the patients were first evaluated at departments of physical medicine, where physicians often were less involved in the NORDSTEN study. If surgery was considered a possible choice of treatment, patients were referred to an orthopedic/neurosurgical department for further consultation. Due to this practice many patients were not screened or included in the OC study.
External validity
NORDSTEN recruited a higher proportion of patients and hospitals than, e.g., the SPORT study [16, 17] and to our knowledge, any other former published randomized LSS trials. In addition, dropout rates have been very low; both at 2 years postoperatively (90%) and indications for 5 years postoperatively (> 80%).
The duration of symptoms for patients operated in the NORDSTEN study was greater than 1 year for the majority of patients (leg/back pain: 71%/81%), considerably higher than in the SPORT study where only about one third had symptoms for greater than 1 year [16]. The Scandinavian tradition is to let patients recover through natural course via nonsurgical treatment and offer those who do not benefit surgery. Therefore, our population varies somewhat from the population reported in the SPORT trials; however, the change in ODI from baseline to 2 year follow-up is comparable to the SPORT study [14, 15, 17, 18]. The high proportion of female patients operated for DS was in accordance with other studies [16, 19, 20].
Ideally, all consecutive eligible patients should have been enrolled in the NORDSTEN study. Since this was not the case, a corresponding patient population from the NORspine registry provided useful additional information about those not included. The NORspine national coverage rate at institutional and individual level varies throughout the years and hospitals. The report from 2019 stated a coverage rate of 95% at the institutional level and 69% at the individual level for lumbar spine surgery [21]. Dropout analysis showed that patients who were not reported to the registry, were mainly emergency patients. The coverage rate for planned surgery was nearly twice as high as for emergency surgery [21]. Therefore, the LSS patients operated and reported to the registry should be representative for the typical LSS patients treated by surgery in Norway, and suitable for comparison with the NORDSTEN-study population. The patients in the NORDSTEN study and the NORspine registry were similar at baseline indicating that results from the NORDSTEN study may be generalized to the broader population. Although a large study population alone cannot exclude selection bias the pragmatic nature of NORDSTEN may also generate evidence generalizable to routine practice.
Even though the patients enrolled in the NORDSTEN study were found to be representative for the Norwegian surgical LSS-population, this may not be the case elsewhere. However, the population characteristics of NORspine have been found to be similar in other Scandinavian countries [22], and also comparable to LSS populations in USA [23]. Baseline demographical data in the two randomized NORDSTEN trials are in accordance with former studies both regarding age, gender, body mass index, ODI score, leg pain and health-related quality of life [16, 17, 19, 20].
Feasibility
Several factors potentially contributed to the feasibility of this triple designed, multicenter study with complex protocols. With several study hospitals and multiple study personnel involved, communication was at the forefront of the design. There was a common agreement on the importance of achieving a higher level of scientific evidence to guide clinical decisions that may have been vital in the present project. In addition, study hospitals were supported in the adaption of the study’s procedures to their local routines through initiation meetings. The biannual meetings held throughout the study period for all study governance groups encouraged dialog and sharing of experiences between all groups which had an important sub goal; to achieve a sense of ownership to the project throughout all organizational levels. In addition, all regional PIs were represented on the scientific board contributing to effective dissemination of information within the study network. Another potential beneficial factor contributing to the high and timely recruitment rate, may be the parallel recruitment in all three trials.
The implementation of GCP ensured an ethical and scientific quality of data collection and patient follow-up, even though also contributing to increasing trial complexity and costs [24].
The NORDSTEN study applied for and has been granted public financial support from the health authorities that gave the possibility to implement this large multicenter study independently.
The Norwegian health care system is founded on the principle of universal access regardless of differences in socioeconomic status and place of living. The responsibility for provision of health care is decentralized. Differences in health care systems could influence the choice of treatment of LSS patients and spine surgery compared to countries with higher share of nonpublic health services and hospitals primarily funded by private insurance. The Norwegian national identity number provides the opportunity to follow-up patients nationwide.
Future plans for the NORDSTEN study
The NORDSTEN 5 year follow-up ends in 2023 and 10 year in 2028, and several publications are planned regarding both clinical and radiological outcomes of these LSS patients treated surgically or nonsurgically. The high follow-up rate provides a good base for further and deeper investigations into the clinical and radiological outcomes. Other publications alongside the main study results are among others planned regarding predictor analysis, methodological analysis and cost analysis.
Conclusion
The NORDSTEN study provide important evidence regarding surgical and nonsurgical treatment of patients with LSS. Baseline data from the NORspine registry suggests that patients enrolled in the NORDSTEN study are similar to LSS patients treated in routine surgical practice.
Availability of data and materials
The datasets generated during the current study are available from the corresponding author on reasonable request. Medical researchers’s request has to be in accordance with local registration and ethical approval, and datasets will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. All proposals requesting data access will need approval of the scientific board before any data can be released.
Abbreviations
- ASA:
-
American society of anesthesiologists classification
- CRF:
-
Case report forms
- DA:
-
Decompression alone
- DF:
-
Decompression with instrumental fusion
- DS:
-
Degenerative spondylolisthesis trial
- FORMI:
-
Research and communication unit for musculoskeletal health
- GCP:
-
Good clinical practice
- LSS:
-
Lumbar spinal stenosis
- NORDSTEN:
-
Norwegian Degenerative spondylolisthesis and spinal stenosis study
- NORspine:
-
Norwegian Registry for Spine Surgery
- NRS:
-
Numeric rating scale
- OC:
-
Observational cohort
- ODI:
-
Oswestry disability index
- PI:
-
Principal investigator
- PROM:
-
Patient reported outcome measures
- SST:
-
Spinal stenosis trial
References
Kim CH et al (2018) Increased volume of surgery for lumbar spinal stenosis and changes in surgical methods and outcomes: a nationwide cohort study with a 5 year follow-up. World Neurosurg 119:e313–e322
Grøvle L et al (2019) The rates of LSS surgery in Norwegian public hospitals: a threefold increase from 1999 to 2013. Spine 44(6):E372-e378
Machado GC et al (2017) Trends, complications, and costs for hospital admission and surgery for lumbar spinal stenosis. Spine 42(22):1737–1743
Minamide A, Yoshida M, Maio K (2013) The natural clinical course of lumbar spinal stenosis: a longitudinal cohort study over a minimum of 10 years. J Orthop Sci 18(5):693–698
Adamova B et al (2015) Outcomes and their predictors in lumbar spinal stenosis: a 12 year follow-up. Eur Spine J 24(2):369–380
Machado GC et al (2016) Surgical options for lumbar spinal stenosis. Cochrane database syst rev 11(11):cd012421
Deyo RA et al (2010) Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA 303(13):1259–1265
Weiss AJ, Elixhauser A, Andrews RM (2011) Characteristics of Operating Room Procedures in U.S. Hospitals, 2011: Statistical Brief #170. In: Healthcare cost and utilization project (HCUP) statistical briefs. Agency for healthcare research and quality (US): Rockville (MD)
Hermansen E et al (2017) Study-protocol for a randomized controlled trial comparing clinical and radiological results after three different posterior decompression techniques for lumbar spinal stenosis: the spinal stenosis trial (SST) (part of the NORDSTEN study). BMC Musculoskelet Disord 18(1):121
Austevoll IM et al (2019) Decompression alone versus decompression with instrumental fusion the NORDSTEN degenerative spondylolisthesis trial (NORDSTEN-DS); study protocol for a randomized controlled trial. BMC Musculoskelet Disord 20(1):7
Lee S et al (2010) A practical MRI grading system for lumbar foraminal stenosis. AJR Am J Roentgenol 194(4):1095–1098
ICH (2016) International council for harmonisation of technical requirements for pharmaceuticals for human use. ICH harmonised guideline. Integrated addendum to ICH E6(R1): Guideline for good clinical practice. E6 (R2)
Grotle M, Brox JI, Vollestad NK (2003) Cross-cultural adaptation of the Norwegian versions of the roland-morris disability questionnaire and the oswestry disability index. J Rehabil Med 35(5):241–247
Austevoll IM et al (2021) Decompression with or without fusion in degenerative lumbar spondylolisthesis. N Engl J Med 385(6):526–538
Hermansen E et al (2022) Comparison of 3 different minimally invasive surgical techniques for lumbar spinal stenosis: a randomized clinical trial. JAMA Netw Open 5(3):e224291
Cummins J et al (2006) Descriptive epidemiology and prior healthcare utilization of patients in the spine patient outcomes research trial’s (SPORT) three observational cohorts: disc herniation, spinal stenosis, and degenerative spondylolisthesis. Spine 31(7):806–814
Weinstein JN et al (2009) Surgical compared with nonoperative treatment for lumbar degenerative spondylolisthesis. Four year results in the spine patient outcomes research trial (SPORT) randomized and observational cohorts. J Bone Joint Surg Am 91(6):1295–1304
Weinstein JN et al (2008) Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med 358(8):794–810
Försth P et al (2016) A randomized, controlled trial of fusion surgery for lumbar spinal stenosis. N Engl J Med 374(15):1413–1423
Ghogawala Z et al (2016) Laminectomy plus fusion versus laminectomy alone for lumbar spondylolisthesis. N Engl J Med 374(15):1424–1434
Solberg TK, Olsen LR, Tyrihaug AM (2020) Annual report 2019. In: Norwegian registry for spine surgery (NORspine)
Lønne G et al (2019) Lumbar spinal stenosis: comparison of surgical practice variation and clinical outcome in three national spine registries. Spine J 19(1):41–49
Lønne G et al (2017) Variation in selection criteria and approaches to surgery for lumbar spinal stenosis among patients treated in Boston and Norway. Clin Neurol Neurosurg 156:77–82
Mentz RJ et al (2016) Good clinical practice guidance and pragmatic clinical trials: balancing the best of both worlds. Circulation 133(9):872–880
Acknowledgements
We would like to thank the patients who volunteered for this study, to Inger Ljostad, our patient representative, for her involvement in implementing the study, and to Katarina Mølsæter (Data and Safety Monitoring Board, Møre and Romsdal Health Trust) for her dedication and her diligent review of the data. We gratefully acknowledge the contributions of Bodil Røyset and Anniken Remseth in managing the trial finances. From the NORDSTEN scientific board we thank, Helena Brisby, Clemens Weber, Eric Franssen, Oliver Grundnes, Hasan Banitalebi, Jørn Aaen and Masoud Anwar. Thank you to the following members of the NORDSTEN working group: Turid Rognsvåg, Janne Haugland, Anita Karin Vassbakk, Vidar Punsvik, Kirstine Eikenes, Therese Gundersen, Trine Myrvold, Sara Søreng, Silje Nilsen, Arild Hjulstad, Grete Ward, Turid Fjesme, Nikolaos Ikonomou, Ove Bjørnstad, Yngve Sporstøl, Ted P. Lundgren, Anne-Charlotte Fosse Haug, Elisabeth Lilleholdt Muller, Kjartan Krogedal, Bettina Timenes, Vidar Opland, Merete Finjarn, Håvard Furunes, Hege Bergum Nilsen, Samer Habiba, Kristine Helland, Maria Rieber-Mohn, Andreas Seip, Greger Lønne, Gisle Szacinski, Espen Sjåberg, Ellen Aksnes, Øystein Nygaard, Hege Andresen, Andreas Sørlie, Terje Fallås, Paal Arnesen, Kristine Evanger, Knut Jørgen Haug, Ingrid Edborg, Arnfinn Pedersen, Hege Westgard, Kamaran Raza, Odd Arild Ågedal and all other local study coordinators and surgeons not named her who contributed to the realization of this trial. A special thanks to the staff at The Research and Communication Unit for Musculoskeletal Health (FORMI), Oslo University Hospital, for meticulous work with data collection (Eira Kathleen Ebbs, Marie Skovli Pettersen, Ørjan Nesse Vigdal, Fiona Aanesen).
Funding
Open access funding provided by University of Bergen (incl Haukeland University Hospital). This work has been supported by grants from The Western Norway Regional Health Authority, The Central Norway Regional Health Authority and the Møre and Romsdal Hospital Trust. The funding authorities were not involved in the study design, collection or analysis of data, interpretation of the results or drafting of the manuscript.
Author information
Authors and Affiliations
Contributions
KI, EH, IMA, FR, TKS, JIB, CH and KS contributed to the planning of the study; IFB and MHG contributed to collect the data; KI, IFB and KS analyzed the results, interpreted the results and wrote the draft of the manuscript. All authors contributed to revision of the draft and approved the final version to be published.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or nonfinancial interest to disclose.
Ethics approval
The Norwegian Committees for Medical and Health Research Ethics approved the trials (NORDTEN-SST trial and NORDSTEN-OC; 2011/2034, NORDSTEN-DS trial; 2013/366). Data protection was approved by the Norwegian Data Inspectorate and data are stored at “Services for sensitive data” (TSD) hosted by the University of Oslo, to allow secure data sharing between researchers. The study was performed according to the Helsinki Declaration and registered at ClinicalTrials.gov under the following identifiers; SST: NCT02007083 10/12/2013 / DS: NCT02051374 31/01/2014/OC: NCT03562936 20/06/2018. The trial is monitored according to requirements of the Good Clinical Practice (GCP) guidelines.
Consent to participate
Informed consent was obtained from patients included in the study after oral and written information.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Indrekvam, K., Bånerud, I.F., Hermansen, E. et al. The Norwegian degenerative spondylolisthesis and spinal stenosis (NORDSTEN) study: study overview, organization structure and study population. Eur Spine J 32, 4162–4173 (2023). https://doi.org/10.1007/s00586-023-07827-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-023-07827-w