Abstract
Background
Recent signs of fraudulent behaviour in spine RCTs have queried the integrity of trials in the field. RCTs are particularly important due to the weight they are accorded in guiding treatment decisions, and thus, ensuring RCTs’ reliability is crucial. This study investigates the presence of non-random baseline frequency data in purported RCTs published in spine journals.
Methods
A PubMed search was performed to obtain all RCTs published in four spine journals (Spine, The Spine Journal, the Journal of Neurosurgery Spine, and European Spine Journal) between Jan-2016 and Dec-2020. Baseline frequency data were extracted, and variable-wise p values were calculated using the Pearson Chi-squared test. These p values were combined for each study into study-wise p values using the Stouffer method. Studies with p values below 0.01 and 0.05 and those above 0.95 and 0.99 were reviewed. Results were compared to Carlisle’s 2017 survey of anaesthesia and critical care medicine RCTs.
Results
One hundred sixty-seven of the 228 studies identified were included. Study-wise p values were largely consistent with expected genuine randomized experiments. Slightly more study-wise p values above 0.99 were observed than expected, but a number of these had good explanations to account for that excess. The distribution of observed study-wise p values was more closely matched to the expected distribution than those in a similar survey of the anaesthesia and critical care medicine literature.
Conclusion
The data surveyed do not show evidence of systemic fraudulent behaviour. Spine RCTs in major spine journals were found to be consistent with genuine random allocation and experimentally derived data.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Randomized controlled trials (RCTs) have been an important tool in evaluating medical interventions since they were introduced by Sir Austin Bradford Hill in 1948 [1, 2]. RCTs are based on random allocation to treatment arms which aims to remove confounding and more accurately estimate the effect of interventions [3]. RCTs have significant weight in guiding treatment decisions and as such, factors undermining their reliability are fundamentally damaging to science [4]. Fabricated or manipulated data can distort the evidence base for a treatment, especially where fraudulent data change the results of a meta-analysis [5, 6]. The public is impacted regardless of whether treatment effects are falsely exaggerated or minimized: patients could be denied life-saving or life-changing treatment; or alternately be exposed to danger with no benefit.
A 2022 article by O’Connell et al. [7] identified a set of trials suspected to present fabricated data for the effectiveness of cognitive therapy for back pain. The claimed positive effect for the treatment was markedly superior to any other studies in the field, to the point of possibly changing the conclusions of a meta-analysis. [8] Such academic scandals have seemed few and far between in spine, in particular, compared to other fields such as anaesthesia [9] and more recently, infectious diseases such as COVID-19 [10]. Researchers such as John Carlisle [9, 11] have taken to reviewing trial data integrity in their fields through statistical analyses. These have identified a number of fraudulent trials and have inspired others to do the same for their fields [12, 13].
Carlisle’s method, which is based on the Fisher–Stouffer method [14] (also sometimes referred to as the Stouffer Z-score method) [7], now dubbed the Carlisle–Stouffer method [15], focuses on the extent of the similarity between the trial arms, using patient characteristics at baseline. As arm allocation in RCTs is random, one would expect a uniform distribution of p values between 0 and 1, where studies with a p value approaching 0 have very dissimilar and studies with a p value approaching 1 have very similar groups at baseline and 0.5 neither more similar nor dissimilar than expected. This method uses baseline summary data (either frequency or interval) which is reported in most trials and allows the computation of a ‘study-wise’ p value.
The purpose of this research, by applying methods similar to Carlisle’s, is to broadly assess the probity of RCTs being published in spine journals, to ascertain whether the incident noted by O’Connell et al. [7] was isolated or whether baseline characteristic manipulation was common in spine research. It should be noted that this type of analysis cannot accurately distinguish between intentional misconduct and genuine mistakes. It also cannot detect all fraudulent behaviour but attempts to uncover one of the most common signs of fraud, an abnormal baseline similarity between groups. This is a method that was used by O’Connell and team to uncover a major fraud scandal in spine research. This is not to say that a high study-wise p value is necessarily proof of fraud. Misconduct must be the conclusion only if no other explanations exist for the results obtained.
We chose to examine frequency data alone at this stage due to some spine journals enforcing a low number of decimal places, or integer reporting only, for interval data; and this would bias the study-wise p values towards 1. For example, in a trial with 200 patients per arm, a mean age of 50 and a standard deviation of 10, enforcing mean age reporting as integer-only will result in a p value greater than 0.99 approximately 70% of the time, simply due to the rounding to integer values.
Methods
Search strategy, inclusion criteria and data extraction
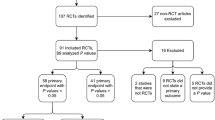
A PRISMA [16,17,18] flowchart is presented in Fig. 1. The PubMed electronic database was searched for articles categorized as RCTs in their PubMed metadata published between 2016 and 2020 in four major spine journals (Spine, The Spine Journal, Journal of Neurosurgery Spine, and the European Spine Journal). Each record was reviewed by a single person between June and July 2022 and papers were excluded if the baseline variables were not separately reported by arm. Baseline patient characteristic data were single-extracted from trials which reported at least one baseline frequency variable. A list of all included papers is in Appendix A in supplementary material. Only baseline categorical data (sometimes referred to as frequency variables, where distinct groups are identified, e.g. male/female), recorded before randomization, were included in this analysis.
Variable-wise p values
The significance of the difference between the arms for each variable was determined using a Pearson Chi-squared test. This test was used regardless of sample size and number of events. While some other tests are preferred in the setting of a small number of events, we prioritized the use of a single test for consistency and comparability. p values were calculated for all variables extracted in the previous step. p values reported in the articles were not included here.
Study-wise p values
A Z score was calculated for each p value obtained previously, with p values of 1 assigned a Z score of 3. For each study, the sum of Z scores was divided by the square root of the number of variables included to obtain a study-wise p value. All studies with a p value below 0.05 and 0.01, as well as those above 0.95 and 0.99, were noted, and reasons for these p values were investigated.
Statistical analysis
The significance of the difference between the observed and expected number of studies for each p value range was calculated using an exact binomial probability. The distributions of variable-wise and study-wise p values were plotted using Microsoft Excel v16.67.
Results
Dataset
A total of 228 articles were first identified in the database search (Fig. 1). Sixty-one of those were excluded for reasons including not containing categorical data, not presenting baseline data separately by arm or having been misclassified as an RCT, among others. One hundred sixty-seven articles were retained for analysis, containing a total of 921 categorical variables, for an average 5.5 variables per study. Figure 2 shows the distribution of the number of variables per study.
Observed p values
A supplementary file containing all variable-wise and study-wise p values is available as Appendix B. Figure 3A and B shows, respectively, all study-wise and variable-wise p values, ordered and plotted according to their percentile. Both sets of values show a near-uniform distribution, as would be expected in the setting of genuine random allocation without data fabrication. Only a small tail towards p values of 1 (Fig. 3B) was observed. The mean variable-wise p values were 0.52, and the mean study-wise p value was 0.58.
A The observed distribution of 921 variable-wise p values across 167 trials, plotted by percentile rank. B The observed distribution of study-wise p values in 167 trials, plotted by percentile rank. The distributions observed are close to the flat distributions expected if all studies report data from genuine randomized experiments. In the setting of systemic fabricated data or non-random allocation, an S shaped curve would be expected
Statistical findings
The number of trials with study-wise p values less than 0.01, less than 0.05, and more than 0.95 were not greater than expected by random chance. Only in the p > 0.99 category did we observe a significant difference between the observed and expected number of studies: only two were expected, but five were identified (p = 0.03) (see Table 1).
Comparison to anaesthesia and critical care
The findings of this study were compared against those of Carlisle’s survey of anaesthesia and critical care in 2017 [11]. In all ‘outlying’ categories, spine presented a lower percentage of RCTs than anaesthesia and critical care (by 2.18%, 2.33%, 1.31% and 0.58% for p < 0.01, p < 0.05, p > 0.95 and p > 0.99, respectively) as can be seen in Fig. 4.
Percentage of p values below 0.01 and 0.05 as well as above 0.95 and 0.99 in anaesthesia and critical care RCTs (dark grey), as reported by Carlisle (2017) and spine RCTs (light grey) compared against the expected percentage (black). In all outlying categories, the proportion of spine RCT study-wise p values is similar to or less than that observed in Carlisle
Discussion
Our results are reassuring in that they do not identify evidence of widespread data fabrication or non-random allocation in published randomized controlled trials, at least in the major spine journals examined.
Explanation for three of the five studies with high p values (p > 0.99)
We did not find more papers with very low p values (suggestive of non-random allocations) than statistically expected (Table 1). A slight preponderance of papers with a p value greater than 0.99 (suggesting groups more similar than expected by simple random allocation) was seen. Three of the five studies with a study-wise p value above 0.99 had potential explanations. The first was a CBT trial from the Monticone group, whose work has been thoroughly impugned and was the trigger for initiating this study [7, 19]. Another study had allocated patients to arms using block randomization across different countries, which is equivalent to blocking at a centre level and causes de facto stratification [20]. A third trial had a considerable discontinuity effect from its small number of patients (15 in each arm) [21]. To account for this small population, we conducted a sensitivity analysis by calculating the variable-wise mid-p and found a study-wise p value of 0.91, which no longer qualifies for the ‘above 0.99’ range. When these three trials are removed from the results, the p value for the difference between the observed and expected number of trials with a study-wise p value above 0.99 is no longer significant (0.5).
Why is there less observed fraud in spine research than in other specialties?
The prevalence of ‘abnormal’ p values appears to be low in spine RCTs. This is certainly true when compared to anaesthesia (see Fig. 4). It is not possible to provide a definitive explanation for the observed differences between research areas; however, we would speculate about three possible causes. Firstly, there are very few single-surgeon trials in spine research, which may be preventing incidents such as the Fujii scandal [9], where an anaesthetist could claim to see thousands of patients a year, without arousing too much suspicion. Secondly, there may be less pressure on spine clinicians to publish as a tool for career advancement than in some other specialties. Thirdly, many spine conferences and journals are accepting of trials with null or negative findings as a substantive contribution to the literature which may remove some perverse incentives and career risks associated with relying on the inherently uncertain nature of genuine clinical study outcomes.
Limitations
As mentioned earlier, this analysis is not capable of detecting all fraud and results produced by this study should not be taken as accusations of fraud. Where a trial is genuinely conducted, but outcome data are modified after collection, we would not expect any abnormalities to be detected by this method. This method will detect fraud, provided it presents as non-random allocation, but will not detect weaknesses in study design or interpretation.
Furthermore, our results may not be generalizable. The inclusion criteria limited the analysis to ‘reputable’ journals and the findings presented here may not apply to potentially ‘predatory’ journals, which are associated with a lower quality of research and peer review [7].
Conclusion
We conclude that overall, baseline data in RCTs published in major spine journals are consistent with genuine random allocation. No evidence of systemic suspicious activity was detected. Readers can thus, while remaining cautious, generally assume that RCTs published in these spine journals are genuine.
References
Hyun SJ, Kim KJ, Jahng TA, Kim HJ (2017) Minimally invasive robotic versus open fluoroscopic-guided spinal instrumented fusions: a randomized controlled trial. Spine 42(6):353–358
Solomon MJ, McLeod RS (1998) Surgery and the randomised controlled trial: past, present and future. Med J Aust 169(7):380–383
Roberts C, Torgerson D (1998) Understanding controlled trials: randomisation methods in controlled trials. BMJ 317(7168):1301–1310
Li W, van Wely M, Gurrin L, Mol BW (2020) Integrity of randomized controlled trials: challenges and solutions. Fertil Steril 113(6):1113–1119
Lawrence JM, Meyerowitz-Katz G, Heathers JAJ, Brown NJL, Sheldrick KA (2021) The lesson of ivermectin: meta-analyses based on summary data alone are inherently unreliable. Nat Med 27(11):1853–1854
Hill A, Garratt A, Levi J, Falconer J, Ellis L, McCann K et al (2021) Retracted: meta-analysis of randomized trials of ivermectin to treat SARS-CoV-2 infection. Open Forum Infect Dis 8(11):358
O’Connell NE, Moore RA, Stewart G, Fisher E, Hearn L, Eccleston C et al (2022) Investigating the veracity of a sample of divergent published trial data in spinal pain. Pain. https://doi.org/10.1097/j.pain.0000000000002659
de C Williams AC, Fisher E, Hearn L, Eccleston C (2020) Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane pain, palliative and supportive care group, editor. Cochrane Database Syst Rev 2021(11). https://doi.org/10.1002/14651858.CD007407.pub4
Carlisle JB (2012) The analysis of 168 randomised controlled trials to test data integrity. Anaesthesia 67(5):521–537
Thorlund K, Sheldrick K, Meyerowitz-Katz G, Singh S, Hill A (2022) Making statistical sense of the molnupiravir MOVe-OUT clinical trial. Am J Trop Med Hyg 106(5):1301–1304
Carlisle JB (2017) Data fabrication and other reasons for non-random sampling in 5087 randomised, controlled trials in anaesthetic and general medical journals. Anaesthesia 72(8):944–952
Yentis SM (2012) Lies, damn lies, and statistics*. Anaesthesia 67(5):455–456
Bolland MJ, Avenell A, Gamble GD, Grey A (2016) Systematic review and statistical analysis of the integrity of 33 randomized controlled trials. Neurology 87(23):2391–2402
Stouffer SA, Suchman EA, DeVinney LC, Star SA, Williams Jr RM (1949) The american soldier: adjustment during army life (studies in social psychology in world war II) vol 1
Mascha EJ, Vetter TR, Pittet JF (2017) An appraisal of the carlisle-stouffer-fisher method for assessing study data integrity and fraud. Anesth Analg 125(4):1381–1385
Haddaway NR, Page MJ, Pritchard CC, McGuinness LA (2022) PRISMA2020: an R package and shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and open synthesis. Campbell Syst Rev 18(2):1230. https://doi.org/10.1002/cl2.1230
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 88:n71
Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ 372:n160
Monticone M, Ambrosini E, Cazzaniga D, Rocca B, Motta L, Cerri C et al (2016) Adults with idiopathic scoliosis improve disability after motor and cognitive rehabilitation: results of a randomised controlled trial. Eur Spine J 25(10):3120–3129
Fischgrund JS, Rhyne A, Franke J, Sasso R, Kitchel S, Bae H et al (2018) Intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: a prospective randomized double-blind sham-controlled multi-center study. Eur Spine J 27(5):1146–1156
Coronado RA, Devin CJ, Pennings JS, Vanston SW, Fenster DE, Hills JM et al (2020) Early self-directed home exercise program after anterior cervical discectomy and fusion: a pilot study. Spine 45(4):217–225
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This study did not receive any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have conflicts relevant to this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Levayer, M.M.S., Chew, G.R.P., Sheldrick, K.A. et al. Characteristics of baseline frequency data in spinal RCTs do not suggest widespread non-random allocation. Eur Spine J 32, 3009–3014 (2023). https://doi.org/10.1007/s00586-023-07813-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-023-07813-2