Abstract
Objective
The purpose of this study was to systematically review the evidence on inflammatory biomarkers as analytic predictors of non-specific low back pain (NsLBP).
Summary of background data
Low back pain (LBP) is the number one cause of disability globally, posing a major health problem that causes an enormous social and economic burden, and there is an increasing interest on the importance of biomarkers in quantifying and even emerge as potential therapeutic tools to LBP.
Methods
A systematic search was conducted on July 2022 in Cochrane Library, MEDLINE and Web of Science for all the available literature. Cross-sectional, longitudinal cohort or case–control studies that evaluated the relationship between inflammatory biomarkers collected from blood samples and low back pain in humans were considered eligible for inclusion, as well as prospective and retrospective studies.
Results
The systematic database search resulted in a total of 4016 records, of which 15 articles were included for synthesis. Sample size comprised a total of 14,555 patients with LBP (acute LBP (n = 2073); chronic LBP (n = 12482)) and 494 controls. Most studies found a positive correlation between classic pro-inflammatory biomarkers and NsLBP, namely C-reactive protein (CRP), interleukin 1 (IL-1) and IL-1β, interleukin 6 (IL-6) and tumour necrosis factor α (TNF-α). On the other hand, anti-inflammatory biomarker interleukin 10 (IL-10) demonstrated a negative association with NsLBP. Four studies have made direct comparisons between ALBP and CLBP groups regarding their inflammatory biomarkers profile.
Conclusions
This systematic review found evidence of increased levels of pro-inflammatory biomarkers CRP, IL-6 and TNF-α and decreased levels of anti-inflammatory biomarker IL-10 in patients with LBP. Hs-CRP was not correlated with LBP. There is insufficient evidence to associate these findings with the degree of pain severity or the activity status of the lumbar pain over time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Low back pain (LBP) is now the number one cause of disability globally [1], posing a major health problem that causes an enormous social and economic burden on the community and health systems [2, 3]. It has a calculated global prevalence of 9442.5 per 100,000 adults (9%) [4]. Chronic low back pain (CLBP) is defined as pain, muscle tension or stiffness localized below the costal margin and above the inferior gluteal folds, with or without neurological symptoms in the lower limb, and is defined as chronic when it persists for 12 weeks or more [5]. CLBP affects approximately 20–25% of the elderly population (older than 65 years) [6,7,8]. At any given time, 12–33% of the adult population has low back pain [9], being more common in women than in men [10,11,12]. Causes of CLBP can be distinguished into specific (e.g. degenerative process to the spinal segments of the lumbar spine such as lumbar spinal stenosis, spondylolisthesis, disc degeneration or herniation) or non-specific, when there is no identifiable cause of pain [13,14,15,16].
Cytokines are regulatory proteins (pro-inflammatory biomarkers) which, in the case of inflammation, modulate the inflammatory response of all cells of the immune system. Pro-inflammatory cytokines, such as IL-1B, IL-6 and TNF-α, can be objectively measured in the CNS and circulation and have been implicated in the processes of central sensitization and chronic LBP [16,17,18]. On the other hand, anti-inflammatory cytokines, such as IL-4 and IL-10, inhibit pro-inflammatory cytokine response. Elevated IL-6 and reduced IL-10 levels were described in peripheral blood of non-specific chronic low back pain (NsCLBP) patients, thereby suggesting that an imbalance between pro-inflammatory and anti-inflammatory mediators contributes to the pathophysiology of LBP [19, 20]. Other studies reported an association between increased pro-inflammatory cytokines and pain intensity levels in a population with NsCLBP [21,22,23,24,25,26].
The purpose of this study was to systematically review the evidence on inflammatory biomarkers as analytic predictors of NsLBP, assess whether patients with NsLBP present changes in several inflammatory biomarkers and analyse whether LBP severity is associated with the magnitude of changes in inflammatory biomarkers. A deeper understanding of the aetiology and pathophysiology associated with non-specific LBP may lead to a better stratification of these patients and to the development of better targeted interventions.
Materials and methods
Search strategy and selection criteria
The recommendations from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [27] were followed to perform this systematic review, and its protocol was registered on the International prospective register of systematic reviews (PROSPERO—ID CRD42022375174).
A systematic search was conducted on July 2022 in Cochrane Library, MEDLINE and Web of Science for all the available literature, based on the following query: (“low back pain” OR “lower back pain” OR “back pain” OR “backache” OR "back ache" or “lumbago” OR “lumbar pain” OR “sciatica” OR “back disorder”) AND (“inflammation mediators” OR “inflammatory markers” OR “cytokines” OR “interleukins” OR “monokines” OR “chemokines” OR “tumor necrosis factor” OR “C-Reactive Protein” OR "CRP" OR “C-reactive protein” OR “hs-CRP” OR "hsCRP" OR “biological markers” OR “biomarkers”). Studies were retrieved and duplicates removed electronically and manually. References were transferred to the reference management software Rayyan (https://rayyan.qcri.org). This software was developed specifically to expedite the initial screening of abstracts and titles for systematic reviews and to allow for blinded screening, in this case, between two authors.
Cross-sectional, longitudinal cohort or case–control studies that evaluated the relationship between inflammatory biomarkers and low back pain in humans were considered eligible for inclusion, as well as prospective and retrospective studies.
Literature screening
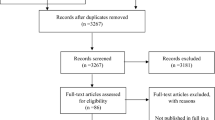
Each full-text article was searched for reports studying a correlation between inflammatory biomarkers and the prevalence and intensity of chronic LBP. Observational cohort studies (with and without control group), cross-sectional studies and randomized clinical trials (RCT) were included. Studies that comprised patients older than 18 years old, in which one or more inflammatory biomarkers were measured in blood plasma, were included. We excluded publications in a population with previous lumbar pathology, with underlying systemic pathology (e.g. autoimmune diseases or osteoarthritis) that could influence the systemic inflammatory biomarkers measurement and studies with fewer than 20 subjects. The duration of LBP was retrieved to distinguish between acute (< 6 weeks) and chronic (≥ 6 weeks) NsLBP, and studies in a population with different sources of pain besides LBP were excluded, as well as studies with evaluation of areas other than the lumbar spine (e.g. cervical and thoracic spine) or evaluating inflammatory biomarkers in the intervertebral discs. Studies specifically evaluating the effectiveness of pharmaceutical intervention on pain or anti-cytokine therapy, biomechanical and cadaver studies, duplicate publications and studies that did not meet the inclusion criteria were also excluded (Fig. 1).
Data extraction
The following items were recorded from all eligible studies: study design and study purpose, patient demographics and related characteristics (number, age, gender, body mass index), inclusion and exclusion criteria, patient outcomes associated with biomarker concentrations and included biomarkers. Data concerning the measuring instrument or scale used to evaluate the severity of LBP, quality of life and return to work were also extracted. The type, number and concentration of the assessed inflammatory biomarkers were extracted, and no particular biomarker associated with changes in inflammatory processes was excluded. Data of titles and abstracts were analysed independently by two reviewers, and any discrepancies were resolved by consensus. After the primary selection, full-text eligibility was done by two reviewers using screening tools developed a priori. The most common limitations amongst the studies were a low number of patients included, lack of ascertainment of exposure or definition of controls, lack of blinding for outcome assessments and adequacy of follow-up of cohorts. We present the results of the cross-sectional studies and the longitudinal studies separately. When odds ratios (ORs) were presented, the p-value and the 95% confidence interval (CI) were extracted. For other measures of association, the p-value was used to assess whether the association was statistically significant.
Assessment of study quality
The methodological quality of included studies was evaluated using two versions (for case–control and cohort studies) of the Newcastle–Ottawa Assessment scale (NOS) [28] (Table 2). As described in Table 2, amongst all studies, the minimum score was 4 out of 9 and maximum score was 7 out of 9, with a median score of 6.
Results
Study selection
The systematic database search resulted in a total of 4016 records (Web of Science (n = 1962), MEDLINE (n = 2051), Cochrane Library (n = 3)). Following deduplication, a total of 3022 potentially relevant studies were identified and screened for retrieval, of which 2831 articles were excluded as they did not fulfil the eligibility criteria. Regarding the selection of articles for full-text screening, there was 95% consensus between the two reviewers, and 191 studies were included, of which 176 were excluded. The reasons for exclusion were as follows: review articles (n = 10), articles with non-serum biomarkers evaluation (i.e. inflammatory biomarkers evaluated by intervertebral disc, ligamentum flavum or muscle biopsy) (n = 33), evaluation of patients with specific spinal disorders (n = 28) and articles that did not meet the inclusion criteria (n = 105). Hence, 15 articles were included for synthesis in this systematic review. The entire selection process is presented in the flowchart of Fig. 1.
Study characteristics
Studies characteristics and demographics are presented in Table 1. All the papers were published between 2006 and 2022, with 12 of the 15 included studies published from 2016 to 2022. Eleven studies [19, 29,30,31,32,33,34,35,36,37,38,39] were cross-sectional and four were longitudinal [40,41,42,43]. Regarding the characteristics of the population included, seven studies [34, 35, 37, 39,40,41,42,43] assessed non-specific acute low back pain (NsALBP) and ten studies [19, 29, 32,33,34, 36,37,38,39,40,41] included persons with NsCLBP. The differentiation cut-off between acute low back pain (ALBP) and CLBP was 6 weeks. Ten studies [19, 29, 32,33,34, 37,38,39,40,41] presented a control group. Regarding the restrictions in patient inclusion, two studies included only female patients [32, 44] and one studied a multi-generation cohort with evaluations at 14, 17, 20 and 22 years [42]. Sample size comprised a total of 14,555 patients with LBP (ALBP (n = 2073); CLBP (n = 12,482)) and 494 controls. Excluding the multi-generation cohort study [42], the mean age ranged from 29 to 71 years. Regarding the most common inflammatory biomarkers evaluated (Table 3), nine studies included C-reactive protein (CRP) or Hs-CRP (high-sensitive C-reactive protein) [30, 34, 36, 38, 40,41,42,43], eight studies included IL-1 and IL-1β [32,33,34, 37,38,39, 41, 43], ten studies included IL-6 [19, 29, 32, 34, 35, 37,38,39, 41, 43], two studies included IL-10 [19, 34] and eight studies included TNF-α [32, 34, 35, 37,38,39, 41, 43]. Other inflammatory biomarkers that were not analysed in more than one study are described in Table 1, but an exhaustive analysis of these was not carried out. Pain severity was evaluated most commonly using the visual analogue scale (VAS) in five studies [32, 34, 37, 39, 40], Roland–Morris Disability Questionnaire (RMDQ) in four studies [32, 34, 41, 43], numerical rating scale (NRS) in three studies [35, 41, 43], McGill Pain Questionnaire (MPQ) in three studies [29, 35, 38] and Oswestry Disability Index (ODI) in two studies [37, 38].
Clinical features in relationship to biomarkers
LBP assessment, duration of symptoms, biomarkers studied, source and technique of collection, analysis of inflammatory biomarkers and the type of association (positive, negative, or non-existent) between clinical parameters and inflammatory biomarkers are presented in Tables 2 and 3. A positive association means that the presence of LBP is associated with higher levels of the pro-inflammatory biomarkers measured in the blood serum. Regarding Hs-CRP, only one study [40] demonstrated a positive association with the presence of LBP and two studies [40, 42] did not find any relation between these variables. In studies that evaluated only C-reactive protein (CRP) [30, 34, 36, 39, 41, 43], all showed a positive association with the presence of LBP. Within the eight studies evaluating interleukin 1 (IL-1) and IL-1β, in seven studies [33,34,35, 37, 39, 41, 43] no association was found with the presence of LBP. On the contrary, in a cross-sectional study including 148 patients with CLBP and 150 controls, Dadkhah et al. [38] found a positive correlation between these variables. The pro-inflammatory biomarker interleukin 6 (IL-6) was assessed in ten studies, and a positive association was found between IL-6 and CLBP in six studies [19, 34, 35, 38, 39, 43]. Klyne et al. [34], in a study comparing a group of 99 patients with ALBP (acute low back pain) (divided into a high and low pain intensity subgroups) with 55 controls, found a positive correlation between IL-6 and patients with high low back pain intensity (p = 0.045), but there were no significant differences between low pain intensity subgroup and control group (p = 0.141). The rest of the studies found no association between these variables [29, 32, 41]. The evaluation of the interleukin 10 (IL-10) biomarker was only assessed in two studies, with a negative association with the presence of LBP. These studies presented a low [19] and moderate [37] risk of bias. The association between changes in serum TNF-α and presence of LBP was achieved in eight studies [32, 34, 35, 37,38,39, 41, 43], with five studies [32, 35, 37, 38, 43] demonstrating a positive association between these variables. Four studies [37, 39,40,41] have made direct comparisons between ALBP and CLBP groups regarding their inflammatory biomarkers profile. Gebhardt et al. [40] found no association between Hs-CRP and acute or chronic LBP. On the other hand, Injeyan et al. [37] demonstrated that IL-1, IL-1β and IL-6 values were significantly higher in the ALBP group (p = 0.0001), but not in the CLBP group, and that TNF-α values were significantly higher in the ALBP group (p = 0.0001) and CLBP group (p = 0.003).
Discussion
There is a lack of evidence regarding the association between inflammatory biomarkers and LBP. In this systematic review, most studies found a positive correlation between classic pro-inflammatory biomarkers and NsLBP, namely CRP (6 out of 6), IL-1 and IL-1β (7 out of 8), IL-6 (6 out of 10) and TNF-α (5 out of 8). On the other hand, Hs-CRP did not present a significant correlation with LBP (1 out of 3) and anti-inflammatory biomarker IL-10 demonstrated a negative association with NsLBP (2 out of 2).
Previous research has demonstrated a link between chronic inflammation and central sensitization development [15, 45]. Khan et al. [46] have demonstrated an association between increasing levels of circulating pro-inflammatory biomarkers, such as CRP and IL-6, and an increase in pain and disc degeneration in a population with specific and NsLBP. In previous reviews, Berg et al. [47] and Lim et al. [48] found consistent evidence for an association between elevated levels of serum or plasma CRP, TNF and IL-6 and the presence of NsLBP. However, the previous two review studies [47, 48] included both acute and chronic non-specific LBP, and the results were not reported separately for each population, which reveals a limitation in the interpretation of results, as these cytokines are reported to play different roles in the acute and chronic phase of pain [43, 49]. Of the included studies that directly compared groups with acute and chronic LBP, Gebhardt et al. [40] have found no differences in Hs-CRP plasma levels between patients with acute and chronic LBP. On the other hand, Injeyan et al. [37] demonstrated increased levels of IL-1, IL-6 and TNF-α in the acute LBP group, but only increased levels of TNF-α in the chronic LBP group.
The characterization of LBP, particularly its intensity and duration, presents important heterogeneity between studies, given the multiplicity of pain assessment questionnaires. However, the assessment through similar evaluation scales (NRS and VAS) is verified in eight studies [32, 34, 35, 37, 39,40,41, 43].
Successful validation of candidate biomarkers is a demanding and time-consuming process that requires multiple rigorous and carefully designed clinical studies. Indeed, there is moderate evidence for the existence of an increase in the pro-inflammatory systemic profile in association with NsLBP. This relationship and the scarce evidence of the association between the severity of NsLBP and the magnitude of changes in the systemic inflammatory profile are matters of extreme importance to be studied in the future. This could provide a set of potential biomarkers that can more objectively monitor the degree of disease activity and become targets for new treatments.
Strengths and limitations
This study presents several strengths, including the use of PRISMA guidelines for reporting systematic reviews and the pre-registration in the PROSPERO database, reducing unplanned duplication and potential publication bias. Contrary to previous reviews, a survey was carried out excluding patients with some type of underlying pathology of the spine or systemic pathology that could influence inflammatory biomarkers measurement. On the other hand, the differentiation between ALBP and CLBP regarding changes in the inflammatory profile was performed and is detailed in Tables 1 and 3. The differentiation between studies evaluating CRP and Hs-CRP was also performed.
This study has some limitations. Studies on this topic are mostly case–control studies, which entail certain intrinsic limitations such as sampling bias and recall bias, in addition to the fact that this type of studies could only reach a level B of evidence at most. In this type of research, a publication bias is also a possibility, given that funnel plot is not possible due to the heterogeneity of markers and publications. On the other hand, screening references may result in an over representation of positive studies, as trials with a negative result are less likely to be published and hence referred. There was a cross-sectional limitation to the studies presented regarding the presentation of mean differences and confidence intervals or biomarker concentration levels. One article [38] did not accurately describe the method for biomarker analysis, which can compromise the quality of the analysis. On the other hand, although the included studies present gender percentages of the included patients and no significant differences were found between control and patient groups for age and gender, the comparative analysis of the differences in systemic cytokine level between males and females was not investigated. On the other hand, the included studies do not differentiate external factors, such as the use of corticosteroids or NSAIDs, which may influence the inflammatory biomarkers value [50].
Finally, previous research [51,52,53] has demonstrated that a significant percentage of patients with ALBP still have some degree of symptomatology after one year of follow-up. Thus, a longer follow-up is advisable in the future studies.
Conclusion
In this systematic review, considering the overall risk of bias of the included studies, individuals with NsLBP were found to have an associated increase of systemic inflammation, demonstrated by the increased levels of pro-inflammatory biomarkers CRP, IL-6 and TNF-α and decreased anti-inflammatory biomarker IL-10. IL-1 and Hs-CRP were not correlated with NsLBP. There is insufficient evidence to associate these findings with the degree of pain severity or the activity status of the lumbar pain over time. The utilization of blood biobanks and longitudinal evaluation of prospective cohorts would be pertinent in examining the relationship between biomarkers and low back pain, as they can provide a valuable resource for obtaining samples and exploring potential mechanisms underlying the association.
References
Collaborators GDaIIaP (2016) Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388(10053):1545–1602. https://doi.org/10.1016/s0140-6736(16)31678-6
Vadala G, Russo F, Battisti S et al (2014) Early intervertebral disc degeneration changes in asymptomatic weightlifters assessed by t1rho-magnetic resonance imaging. Spine 39(22):1881–1886. https://doi.org/10.1097/brs.0000000000000554
Airaksinen O, Brox JI, Cedraschi C, et al. (2006) Chapter 4. European guidelines for the management of chronic nonspecific low back pain. Eur Spine J, 15(Suppl 2):S192–300. doi: https://doi.org/10.1007/s00586-006-1072-1〹
Collaborators GBoDS (2015) Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 386(9995):743–800. https://doi.org/10.1016/s0140-6736(15)60692-4
Vadala G, Russo F, Musumeci M et al (2017) Clinically relevant hydrogel-based on hyaluronic acid and platelet rich plasma as a carrier for mesenchymal stem cells: rheological and biological characterization. J Orthop Res 35(10):2109–2116. https://doi.org/10.1002/jor.23509
Cannata F, Vadala G, Ambrosio L et al (2020) Intervertebral disc degeneration: a focus on obesity and type 2 diabetes. Diabetes Metab Res Rev 36(1):e3224. https://doi.org/10.1002/dmrr.3224
Hoy D, Bain C, Williams G et al (2012) A systematic review of the global prevalence of low back pain. Arthritis Rheum 64(6):2028–2037. https://doi.org/10.1002/art.34347
Hartvigsen J, Hancock MJ, Kongsted A et al (2018) What low back pain is and why we need to pay attention. Lancet 391(10137):2356–2367. https://doi.org/10.1016/s0140-6736(18)30480-x
Walker BF (2000) The prevalence of low back pain: a systematic review of the literature from 1966 to 1998. J Spinal Disord 13(3):205–217. https://doi.org/10.1097/00002517-200006000-00003
Meucci RD, Fassa AG, Faria NM (2015) Prevalence of chronic low back pain: systematic review. Rev Saude Publica. https://doi.org/10.1590/s0034-8910.2015049005874
Wong AY, Karppinen J, Samartzis D (2017) Low back pain in older adults: risk factors, management options and future directions. Scoliosis Spinal Disord 12:14. https://doi.org/10.1186/s13013-017-0121-3
Dunn KM, Hestbaek L, Cassidy JD (2013) Low back pain across the life course. Best Pract Res Clin Rheumatol 27(5):591–600. https://doi.org/10.1016/j.berh.2013.09.007
Hubert MG, Vadala G, Sowa G et al (2008) Gene therapy for the treatment of degenerative disk disease. J Am Acad Orthop Surg 16(6):312–319. https://doi.org/10.5435/00124635-200806000-00003
Maher C, Underwood M, Buchbinder R (2017) Non-specific low back pain. Lancet 389(10070):736–747. https://doi.org/10.1016/s0140-6736(16)30970-9
Woolf CJ (2011) Central sensitization: implications for the diagnosis and treatment of pain. Pain 152(3 Suppl):S2-s15. https://doi.org/10.1016/j.pain.2010.09.030
Sanzarello I, Merlini L, Rosa MA et al (2016) Central sensitization in chronic low back pain: a narrative review. J Back Musculoskelet Rehabil 29(4):625–633. https://doi.org/10.3233/bmr-160685
Brisby H, Olmarker K, Larsson K et al (2002) Proinflammatory cytokines in cerebrospinal fluid and serum in patients with disc herniation and sciatica. Eur Spine J 11(1):62–66. https://doi.org/10.1007/s005860100306
Shamji MF, Setton LA, Jarvis W et al (2010) Proinflammatory cytokine expression profile in degenerated and herniated human intervertebral disc tissues. Arthritis Rheum 62(7):1974–1982. https://doi.org/10.1002/art.27444
Li Y, Liu J, Liu ZZ et al (2016) Inflammation in low back pain may be detected from the peripheral blood: suggestions for biomarker. Biosci Rep 36(4):16. https://doi.org/10.1042/bsr20160187
Wang H, Schiltenwolf M, Buchner M (2008) The role of TNF-alpha in patients with chronic low back pain-a prospective comparative longitudinal study. Clin J Pain 24(3):273–278. https://doi.org/10.1097/AJP.0b013e31816111d3
Licciardone JC, Kearns CM, Hodge LM et al (2012) Associations of cytokine concentrations with key osteopathic lesions and clinical outcomes in patients with nonspecific chronic low back pain: results from the OSTEOPATHIC Trial. J Am Osteopath Assoc 112(9):596–605. https://doi.org/10.7556/jaoa.2012.112.9.596
Teodorczyk-Injeyan JA, McGregor M, Triano JJ et al (2018) Elevated production of nociceptive CC chemokines and sE-selectin in patients with low back pain and the effects of spinal manipulation: a nonrandomized clinical trial. Clin J Pain 34(1):68–75. https://doi.org/10.1097/ajp.0000000000000507
Wang K, Bao JP, Yang S et al (2016) A cohort study comparing the serum levels of pro- or anti-inflammatory cytokines in patients with lumbar radicular pain and healthy subjects. Eur Spine J 25(5):1428–1434. https://doi.org/10.1007/s00586-015-4349-4
Saika F, Kiguchi N, Kobayashi Y et al (2012) CC-chemokine ligand 4/macrophage inflammatory protein-1beta participates in the induction of neuropathic pain after peripheral nerve injury. Eur J Pain 16(9):1271–1280. https://doi.org/10.1002/j.1532-2149.2012.00146.x
Haro H, Shinomiya K, Komori H et al (1996) Upregulated expression of chemokines in herniated nucleus pulposus resorption. Spine 21(14):1647–1652. https://doi.org/10.1097/00007632-199607150-00006
Queiroz BZ, Pereira DS, Rosa NM et al (2015) Functional performance and plasma cytokine levels in elderly women with and without low back pain. J Back Musculoskelet Rehabil 28(2):343–349. https://doi.org/10.3233/bmr-140526
McInnes MDF, Moher D, Thombs BD et al (2018) Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA statement. JAMA 319(4):388–396. https://doi.org/10.1001/jama.2017.19163
Wells GA SB, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P (2011) The Newcastle-Ottowa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute
Heffner KL, France CR, Trost Z et al (2011) Chronic low back pain, sleep disturbance, and interleukin-6. Clin J Pain 27(1):35–41. https://doi.org/10.1097/ajp.0b013e3181eef761
Briggs MS, Givens DL, Schmitt LC et al (2013) Relations of C-reactive protein and obesity to the prevalence and the odds of reporting low back pain. Arch Phys Med Rehabil 94(4):745–752. https://doi.org/10.1016/j.apmr.2012.11.026
Sowa GA, Perera S, Bechara B et al (2014) Associations between serum biomarkers and pain and pain-related function in older adults with low back pain: a pilot study. J Am Geriatr Soc 62(11):2047–2055. https://doi.org/10.1111/jgs.13102
de Queiroz B, Pereira D, Lopes R et al (2016) Association between the plasma levels of mediators of inflammation with pain and disability in the elderly with acute low back pain: data from the back complaints in the elders (BACE)-Brazil study. Spine 41(3):197–203
Luchting B, Heyn J, Woehrle T et al (2016) Differential expression of P2X7 receptor and IL-1β in nociceptive and neuropathic pain. J Neuroinflammation 13(1):100. https://doi.org/10.1186/s12974-016-0565-z
Klyne DM, Barbe MF, Hodges PW (2017) Systemic inflammatory profiles and their relationships with demographic, behavioural and clinical features in acute low back pain. Brain Behav Immun 60:84–92. https://doi.org/10.1016/j.bbi.2016.10.003
Queiroz BZ, Pereira DS, Rosa NMB et al (2017) Inflammatory mediators and pain in the first year after acute episode of low-back pain in elderly women: longitudinal data from back complaints in the elders-Brazil. Am J Phys Med Rehabil 96(8):535–540. https://doi.org/10.1097/phm.0000000000000661
Ho KKN, Simic M, CvancarovaSmåstuen M et al (2019) The association between insomnia, c-reactive protein, and chronic low back pain: cross-sectional analysis of the HUNT study. Norway Scand J Pain 19(4):765–777. https://doi.org/10.1515/sjpain-2019-0033
Teodorczyk-Injeyan JA, Triano JJ, Injeyan HS (2019) Nonspecific low back pain: inflammatory profiles of patients with acute and chronic pain. Clin J Pain 35(10):818–825. https://doi.org/10.1097/ajp.0000000000000745
Dadkhah P, Hashemi SM, Taheri M et al (2020) Association of serum minerals, vitamin D, total protein, and inflammatory mediators and severity of low back pain. Galen Med J 9:e1342. https://doi.org/10.31661/gmj.v9i0.1342
Xu HW, Zhang SB, Yi YY et al (2021) Relationship between vitamin D and nonspecific low back pain may be mediated by inflammatory markers. Pain Physician 24(7):E1015-e1023
Gebhardt K, Brenner H, Stürmer T et al (2006) The course of high-sensitive C-reactive protein in correlation with pain and clinical function in patients with acute lumbosciatic pain and chronic low back pain - a 6 months prospective longitudinal study. Eur J Pain 10(8):711–719. https://doi.org/10.1016/j.ejpain.2005.11.005
Klyne DM, Barbe MF, van den Hoorn W et al (2018) ISSLS PRIZE IN CLINICAL SCIENCE 2018: longitudinal analysis of inflammatory, psychological, and sleep-related factors following an acute low back pain episode-the good, the bad, and the ugly. Eur Spine J 27(4):763–777. https://doi.org/10.1007/s00586-018-5490-7
Beynon AM, Hebert JJ, Beales DJ et al (2021) Multi-trajectory analysis of C-reactive protein and low back pain from adolescence to early adulthood. Eur Spine J 30(4):1028–1034. https://doi.org/10.1007/s00586-020-06677-0
Klyne DM, Barbe MF, Hodges PW (2022) Relationship between systemic inflammation and recovery over 12 months after an acute episode of low back pain. Spine J 22(2):214–225. https://doi.org/10.1016/j.spinee.2021.09.006
Bz Q, Ds P, Nm R et al (2015) Functional performance and plasma cytokine levels in elderly women with and without low back pain. J Back Musculoskelet Rehabil 28(2):343–349
Haddad JJ (2007) On the enigma of pain and hyperalgesia: A molecular perspective. Biochem Biophys Res Commun 353(2):217–224. https://doi.org/10.1016/j.bbrc.2006.12.032
Khan AN, Jacobsen HE, Khan J et al (2017) Inflammatory biomarkers of low back pain and disc degeneration: a review. Ann N Y Acad Sci 1410(1):68–84. https://doi.org/10.1111/nyas.13551
van den Berg R, Jongbloed EM, de Schepper EIT et al (2018) The association between pro-inflammatory biomarkers and nonspecific low back pain: a systematic review. Spine J 18(11):2140–2151. https://doi.org/10.1016/j.spinee.2018.06.349
Lim YZ, Wang Y, Cicuttini FM et al (2020) Association between inflammatory biomarkers and nonspecific low back pain: a systematic review. Clin J Pain 36(5):379–389. https://doi.org/10.1097/ajp.0000000000000810
Sommer C, Kress M (2004) Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett 361(1–3):184–187. https://doi.org/10.1016/j.neulet.2003.12.007
Yan Y, Guo TM, Zhu C (2018) Effects of nonsteroidal anti-inflammatory drugs on serum proinflammatory cytokines in the treatment of ankylosing spondylitis. Biochem Cell Biol 96(4):450–456. https://doi.org/10.1139/bcb-2017-0267
Koleck M, Mazaux JM, Rascle N et al (2006) Psycho-social factors and coping strategies as predictors of chronic evolution and quality of life in patients with low back pain: a prospective study. Eur J Pain 10(1):1–11. https://doi.org/10.1016/j.ejpain.2005.01.003
Melloh M, Elfering A, Egli Presland C et al (2011) Predicting the transition from acute to persistent low back pain. Occup Med (Lond) 61(2):127–131. https://doi.org/10.1093/occmed/kqq194
Starkweather AR, Lyon DE, Kinser P et al (2016) Comparison of low back pain recovery and persistence: a descriptive study of characteristics at pain onset. Biol Res Nurs 18(4):401–410. https://doi.org/10.1177/1099800416631819
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has any potential conflict of interest.
Ethics approval
Since the study concerns a bibliographic review, it does not require approval from the ethics committee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pinto, E.M., Neves, J.R., Laranjeira, M. et al. The importance of inflammatory biomarkers in non-specific acute and chronic low back pain: a systematic review. Eur Spine J 32, 3230–3244 (2023). https://doi.org/10.1007/s00586-023-07717-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-023-07717-1