Abstract
Purpose
The objective of this study was to discuss our experience performing LLIF in the prone position and report our complications.
Methods
A retrospective chart review was conducted that included all patients who underwent single- or multi-level single-position pLLIF alone or as part of a concomitant procedure by the same surgeon from May 2019 to November 2022.
Results
A total of 155 patients and 250 levels were included in this study. Surgery was most commonly performed at the L4–L5 level (n = 100, 40%). The most common preoperative diagnosis was spondylolisthesis (n = 74, 47.7%). In the first 30 cases, 3 surgeries were aborted to an MIS TLIF. Complications included 3 unintentional ALL ruptures (n = 3/250, 1.2%), and 1 malpositioned implant impinging on the contralateral foramen requiring revision (n = 1/250, 0.4%), which all occurred within the first 30 cases. Out of 147 patients with more than 6-week follow-ups, there were 3 cases of femoral nerve palsy (n = 3/147, 2.0%). Two cases of femoral nerve palsy improved to preoperative strength by the 6th week postoperatively, while one improved to 4/5 preoperative strength by 1 year. There were no cases of bowel perforation or vascular injury.
Conclusion
Our single-surgeon experience demonstrates the initial learning curve when adopting pLLIF. Thereafter, we experienced reproducibility in our technique and large improvements in our operative times, and complication profile. We experienced no technical complications after the 30th case. Further studies will include long-term clinical and radiographic outcomes to understand the complete utility of this approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since its first technical description by Ozgur et al. in 2006, multiple studies have been published supporting the utility of the lateral interbody fusion (LIF) technique [1]. Benefits of LIF include increased stability of the spine [2], restoration of foraminal height via large implant placement resulting in indirect decompression [3], and reduced operative time vs. standard open techniques. The larger annular/disc release and subsequent tools and implants allow a reproducible method to restore the degenerative, malaligned spine to a more physiological state [4]. Although the benefits of this fusion are continuously being published, resulting in the increasing popularity of the technique, there have been some limitations in the education and technique adoption. Major shortcomings of the procedure include the need to reposition the patient from the lateral decubitus to the prone position to perform supplemental posterior instrumentation, decompression, and/or osteotomies, the inability to access the L5–S1 level due to the anatomical relationship of the iliac crest to the disc space, and concerns for nerve injuries at the L4–L5 level [5].
In most operating rooms, the flip from lateral decubitus to prone can add significant operative time to the surgery resulting in extended anaesthesia usage and increased hospital costs. Several workarounds have been created to improve efficiency in the lateral position, including standalone interbodies, lateral plate fixation, and unilateral pedicle screws [6, 7]. Although possible, these workarounds have not come into general favour with the larger spine community. There is still a preference to perform pedicle screw placement in the prone position, both for ease and improved lordosis in the prone position [8, 9]. In an effort to restore operating room efficiency while maintaining the benefits of a LIF, the prone lateral technique for interbody fusion is studied [10, 11]. Potential benefits of recent preliminary data have included full access to the posterior column to perform direct decompressions and/or osteotomies and avoiding the intraoperative flip resulting in decreased operative time.
The purpose of this study is to describe our experience with prone lateral interbody fusion. We describe our learning curve, perioperative data, technique modifications, complications, pearls and pitfalls, and suggestions for further refinement of the technique.
Materials and methods
Patient cohort
This is a retrospective, non-randomized controlled study. Patients of all ages who underwent single- or multi-level single-position pLLIF alone or as part of a concomitant procedure by the same surgeon between May 2019 and November 2022 were included. Exclusion criteria were patients that had contraindications for LLIF, including severe osteoporosis, morbid obesity, chronic inflammatory conditions, vascular, visceral, or neural anatomy not compatible with the transpsoas approach, and medical comorbidities preventing surgery clearance.
Definition of single-position pLLIF
Patients included in the study underwent LLIF in the prone position. There was no repositioning of the patient between anterior and posterior spine work, as commonly seen with LLIF in the lateral decubitus position. Discectomy and cage placement were performed via a retroperitoneal transpsoas corridor before percutaneous pedicle screw placement in the majority of cases or open screw placement during realignment surgery.
Surgical technique
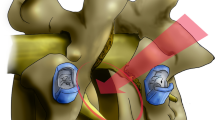
All patients were induced under general anaesthesia and then flipped prone onto a Jackson table with their hips and knees extended and stomachs hanging freely towards the floor. The chest pad was kept in the usual position, while the hip pad was placed more caudally, centred at the greater trochanteric level to improve lateral access at L4–L5. Orthopaedic hip bolsters on the contralateral side of the patient were used for counter pressure during impaction. Skin markings for the lateral incision were made using fluoroscopy along the posterior longitudinal ligament (PLL), anterior longitudinal ligament (ALL), and at the disc angle (Fig. 1).
Fluoroscopy was used to target the surgical level; then, lateral access to the anterior column was obtained via an approximately 2-inch incision parallel to the disc (for single level) or transversely (in multilevel LLIF cases). The obliques were bluntly dissected, and transversalis fascia was penetrated using a tissue dilator or finger; then, finger dissection was used to sweep the retroperitoneal fat. The quadratus lumborum served as the first reference point before dissection was continued until the psoas muscle and transverse process of the surgical level were felt.
After the psoas was located, the initial dilator was docked onto the surface of the muscle, without penetration. The position of the dilator was checked with fluoroscopy before proceeding. The dilator was advanced through the psoas muscle at a ventral to dorsal angle towards the midpoint of the intervertebral disc. Once the disc space is palpated with the dilator, gentle posterior migration is pursued under continuous EMG monitoring and fluoroscopic guidance until the dilator is at the posterior third of the disc space or as posteriorly as neuromonitoring EMG will allow. The angle of the dilator is then straightened to become orthogonal to the disc space.
Sequential dilators were placed over the initial dilator to expand the opening through the psoas and measure retractor blade length. After the appropriate blade lengths are attached to the retractor, the blades are covered using the fingers of a number 9-sized glove (Fig. 2). This prevents retroperitoneal fat from creeping into the surgical field. The retractor is then docked and anchored using a posterior shim. The two largest dilators were removed, but the smallest dilator was kept in place so that it could be used to assess the safety of the quadrants within the retractor via EMG. To ensure safety from unwanted ALL ruptures, an ALL retractor was placed to establish the anterior margin of the disc space for discectomy, endplate preparation, trialling, and final implant placement (Fig. 2). At this point, a safe ventral to dorsal working zone has been established between the ALL retractor and the posterior shim.
Retractor set-up. Top row: retractor blade covered with the fingers of a number 9-sized glove to prevent retroperitoneal fat from creeping into the surgical field. Bottom row: retractor orientation with the ALL retractor in place (left). Anterior/posterior fluoroscopic image of the posterior shim and ALL retractor in place (right)
Discectomy was performed with the surgeon seated and the bed rotated approximately 5–10 degs away from the surgeon. Early in the learning curve, the bed was not rotated to maintain orthogonal to the floor/ceiling. After comfort was achieved. Bed rotation was conducted to have a more ergonomic neck position. The surgeon then proceeded with the annulotomy followed by separation of the disc from the bony endplates using a cobb working in a posterior trajectory towards the posterior shim then levelling out orthogonally after 15–20 mm. Great care was taken to ensure an adequate contralateral release of the bony endplates, which was confirmed with fluoroscopy. Using a boxcutter, the majority of the disc was removed. A large pituitary and ring curette was used to remove the disc followed by a rasp for endplate preparation. Trial implants were then placed under fluoroscopic guidance until desired height and segmental angle were obtained. The trial implant was then replaced with a permanent implant and bone graft. After proper positioning of the implant was seen on fluoroscopy, the retractor was removed. In most cases, the surgeon then proceeded with posterior instrumentation.
Data collection
Patient demographics, including BMI, age, and gender, were collected. Intraoperative data, including estimated blood loss, operative time, retractor time, and complications, were collected. Postoperative data included hospital length of stay and perioperative complications. Postoperative neuropraxia was reported as patients who presented with 3/5 muscle weakness passed the 6-week postoperative mark
Data analysis
Averages, ranges, and standard deviations were obtained from the aforementioned parameters.
Results
Totally, 155 patients and 250 levels were included in this study consisting of 60% (n = 93) females and 40% (n = 62) males. The mean BMI for all patients was 30.4 kg/m2 (range 17.2–50.3 kg/m2). Most patients were over the age of 65 years (63.2%, n = 98), with a range from 17 to 84 years. Postoperative follow-up times include one year (n = 101), 6 weeks (n = 46), 2 weeks (n = 7), and perioperative (n = 1). Long-term postoperative outcome data will be discussed in detail in a separate article.
Surgery was most commonly performed at L4–L5 (n = 100, 40%) and least commonly at T11–T12 (n = 1, 0.4%). Ninety-two (92; 59.4%) single-level surgeries were performed; 71.7% (n=66/92) of single-level surgeries consisted of lateral interbody placement and posterior fusion only, without other surgical procedures at other levels (Table 1). 74.6% (n = 47) of surgeries that consisted of two or more levels included L4–L5. The most common preoperative diagnosis was spondylolisthesis (n = 74, 47.7%) followed by degenerative scoliosis (n = 24, 15.5%). (Figs. 3, 4 and 5).
Retractor times were recorded for most surgeries. The average retractor time was approximately 21 min and ranged from 9 to 38 min (SD = ± 6.5 min). The overall average operative time was 133 min. The average operative time for a single-level surgery was 73 min and ranged from 40 to 108 min (SD = ± 20 min) (Table 2). The overall average estimated blood loss was 171.6 mL. The average estimated blood loss for a single-level surgery level was 43.3 mL and ranged from 20 to 70 mL (SD = ± 15.6 mL). The overall average length of stay for all surgeries was 2.5 days and ranged from 0 to 13 days (SD = 2.1 days). The average length of stay for a single-level surgery was 1.3 days and ranged from 0 to 5 days (SD = ± 1.3 days). Six patients who underwent single-level surgery left on the same day (day 0).
Three (n = 3/155, 1.9%) surgeries were aborted and converted to a minimally invasive (MIS) transforaminal lumbar interbody fusion (TLIF) in the first 30 cases. Complications seen in the first 30 cases included 3 unintentional ALL ruptures (n = 3/250, 1.2%), and 1 malpositioned implant impinging on the contralateral foramen (n = 1/250, 0.4%). We defined postoperative neuropraxia as less than or equal to 3/5 quadriceps strength on the ipsilateral surgical side. Of those patients with at least 6-week follow-up (n = 147) who presented with postoperative neuropraxia, 2 recovered by 6 weeks postop (n = 2/147, 1.4%), and 1 improved to 4/5 strength at 1 year postop (n = 1/147, 0.7%). There were no ALL ruptures, malpositioned implants, femoral nerve palsies, or aborted cases from the 31st case and on. There were no cases of bowel perforation or vascular injury (Table 3, Fig. 6).
Discussion
The purpose of this study is to describe our single-surgeon experience with prone lateral interbody fusion. We describe our learning curve, clinical experience, perioperative complications, indications, and refinement of the technique to reduce complications for the patient and surgeon. Long-term outcome data will be described in detail in a separate article. With growing interest in the surgeon community for single-position spine surgery, understanding the pearls and pitfalls of pLLIF is critical. Emerging technology to improve the reliability of the technique is ongoing, yet surgeon interest and adoption are outpacing approach-specific technology.
Learning curve
Multiple studies have touched upon the steep learning curve to achieve technical proficiency when performing an LLIF. Whether in the lateral decubitus or prone position, the retroperitoneal approach to the spine is unfamiliar to most established spine surgeons. Our team slowly adopted the LLIF technique, and three years after adoption, it has become the workhouse of our practice. Confidence in the technique and subsequent case transition took time to develop. The most common situation one must manage is retractor instability and posterior blade deflection ventrally. Because of this, the authors recommend a prior comfort of the retroperitoneal approach and lateral interbody fusion in the lateral decubitus position before attempting single-position prone lateral interbody fusion. One of the benefits of performing an LLIF in the prone position was having a backup plan in case of unexpected circumstances preventing the completion of the procedure. The backup plan begins in the office with proper surgical planning and foresight. In case of the need to abort, all patients were concomitantly consented and booked for an MIS TLIF. With the TLIF equipment close at hand, our team was sure that a great plan B was within reach. During the first 30 cases, there were three times in which surgeries were aborted. The reasons behind aborting were: (1) unfavourable or unreliable neuromonitoring signals, (2) a long, uncomfortable working distance (the need for > 180 mm blades), and (3) a difficult retractor angle with a high L4–L5 crest. After initial lateral approach, and encountering these issues, these cases were seamlessly and efficiently transitioned from a lateral procedure to an MIS TLIF. Once comfort and proficiency were developed with the technique and tools, no cases were aborted after the 30th case, even though case selection and indications became more complex (multilevel surgery, prone anterior column reconstructions, prone lateral corpectomies). It is our opinion that neuromonitoring is essential to the LLIF procedure and should be used in all cases, especially at the L4–L5 level. Angled instruments were used if a patient presented with a high iliac crest. Long working corridors in patients with high BMIs may be cumbersome and uncomfortable but allowing the abdominal panus to hang freely on a Jackson table moves adipose tissue away from the surgical site. In the following paragraphs, we break the surgical procedure into separate sections and offer an in-depth discussion of our experience.
Indications and contraindications
The indications for patient selection for a pLLIF are continuously expanding. Our most common indication for surgery was spondylolisthesis, followed by degenerative scoliosis. Other indications in our series included adjacent segment disease, degenerative disc disease with foraminal or central stenosis, and spondylodiscitis. It is our opinion that relative contraindications include morbid obesity, and a high iliac crest, while absolute contraindications are abnormal neurovascular anatomy and a ventrally located nerve relative to the disc space within the psoas muscle. The latter two could not be determined without a preoperative MRI, which we recommend for all potential surgical candidates. Special attention should be paid to the axial T2 weighted MRI to view the location of the neurovasculature and their relationship to the operative level to avoid potential vascular injuries. Surgeons should be less hesitant to perform a pLLIF on a patient with a psoas muscle that extends anterior to the vertebral body if the MRI shows a posteriorly located nerve within the muscle. A nerve located anterior to the midpoint of the operative disc space may be a contraindication if it is also noticeably adherent or within a close medial to lateral proximity to the disc space. Lateral and AP x-rays can be used to determine the height of the iliac crest relative to the surgical level, although most high iliac crests can be circumvented to the L4–L5 level by using angled instruments. These instruments may be difficult for surgeons to use early in their learning curve. It is crucial to note that if the surgeon feels that there is a preoperative finding that makes them hesitant to perform a pLLIF, then other forms of interbody fusion that the surgeon is more comfortable with should be pursued. Our experience with pLLIF began with straight ahead trajectories as seen in L2–3 or L3–4 levels. After initial comfort in the prone position was developed, case difficulty increased to include L4–5 s, multilevels, L4–5 s with high crest, proximal lumbar and distal thoracic levels, corpectomies, and ACRs.
Patient positioning
Prone positioning has been documented to play an important role in improving passive segmental lordosis [12] and has been seen to drift the psoas and plexus posteriorly [9]. These are desirable when performing a lumbar interbody fusion using the LLIF technique. Although these benefits have been well defined, optimal patient positioning is crucial and the first challenge we faced. Positioning the patient on a Jackson frame at the highest rung allows the bed to be at a comfortable height so the surgeon may stand for the approach. The Jackson frame also allows the abdominal pannus to hang freely, moving adipose tissue away from the lateral incision site, potentially enabling patients with a higher BMI to undergo pLLIF. The importance of using this table can be seen in this series at the surgeon performed the prone lateral technique on patients with a BMI averaging 30.4 kg/m2 and up to 50.3 kg/m2. There is no specific BMI cut-off; however, a lateral spine–skin distance of less than 180 mm is necessary unless longer retractor blades are available to the surgeon. Taping the lateral flank fat distally can also thin out the flank to help with the approach. We found that distal placement of the hips on the table allowed for more space to access the L4–L5 level while obtaining more lordosis in the lumbar spine. Improved sagittal alignment, especially at L4–L5, is a general goal during fusion. The improved segmental angle seen in prone positioning may decrease the need for further anterior and/or posterior-based releases (ALL release, osteotomies, etc.) to acquire segmental alignment goals. Iatrogenic flat back, even at the segmental level, has been found to increase the risk of accelerated adjacent segment disease and subsequent revision surgery [13].
Bolster and bed rotation
The next challenge was the need to obtain a counterforce to the forces during intervertebral disc work as seen in the lateral decubitus technique. The lateral force applied to a prone patient without a contralateral counterforce can result in patient instability on the table. Bolsters positioned on the contralateral hip over the greater trochanter and against the contralateral lateral chest wall can prevent the patient from sliding away from the surgeon. Pushing the bolster against the patient and light taping ensures intimate contact between the patient and the bolsters. The bolsters help prevent retractor instability during disc work and help prevent the patient from sliding away from the surgeon while rotating the bed for better visualization (Fig. 1). Further considerations for improvements to patient positioning include the use of a formal patient positioner.
Standing for the approach with the bed at xiphoid height is comfortable and allows comfortable shoulder, elbow, and wrist motion to release the retroperitoneal contents. Adequate release of retroperitoneal contents is key to avoiding bowel injuries during the procedure. However, performing disc work at this height would require the surgeon to bend in an uncomfortable crouched position to maintain visualization through the corridor. We have found that performing disc prep while seated was ergonomically more efficient than standing. When first adopting the pLLIF procedure, we feel that keeping the bed parallel to the floor is the right thing to do. It may be easier for the surgeon to conceptualize the proper angle between their instruments and the disc space if they can use the ceiling or floor as a reference angle. This helps eliminate variables as one adopts a new technique. With experience and comfort in the technique, rotation of the table can be incorporated into the surgical workflow. While seated and after the stool height is adjusted, the bed can be rotated 5–10 degs away from the side of the approach until the surgeon does not have to hyperextend their neck to see down the corridor. This allows for improved visualization of the working corridor and better ergonomics for the surgeon. If utilizing bed rotation, the surgeon must be cognizant that their new reference angle changes to the right angle between their instruments and the retractor handles. It is important to note that bed rotation is not necessary to perform a pLLIF, although we feel it has become an integral part of our technique.
Retractor instability and other considerations
One of the foremost challenges when performing a pLLIF is managing retractor instability. The force of gravity and the table mounted A-arm on the retractor in the lateral decubitus position prevents the piston effect on the retractor when malleating or backslapping instruments. In the prone position, the same piston effect can result in losing the retractor position relative to the spine. This instability will happen even when using a posterior shim. Therefore, it is important to keep a close eye on the retractor and posterior shim using fluoroscopy during the procedure to understand if there is retractor migration. Light malleating, light backslapping, and downward pressure on the retractor towards the spine by the assistant are methods to decrease the potential for retractor pullout. Bone screw shims can also be placed into the cranial/caudal blades to anchor the retractor to the spine and maintain retractor stability. These are the same techniques used in traditional LLIF.
The posterior blade of the retractor may deflect (close down) ventrally due to the force of gravity. When using a posterior shim, deflection is more likely to occur once the initial disc material has been removed. A small (2 mm) annular cuff may be helpful to keep the shim in posteriorly. Even with care, the piston effect and ventral migration of the retractor can still occur. If ventral migration of the retractor is not recognized promptly, it could increase the risk of unwanted ALL ruptures or vessel injuries. This is why we placed an ALL retractor during every case at every pLLIF level after the 30th case (Fig. 2). Notably, preoperative axial MRI with vessel to disc space relationship should be studied. Understanding the limits of the anterior disc space eliminated unintentional ALL releases in our series. Furthermore, it allowed for greater opportunity for thorough anterior disc preparation and subsequent segmental lordosis restoration. Potential considerations for retractor modifications include placing a screw that can go through the posterior blade to anchor the retractor to the spine. This would decrease both the retractor instability in the medial–lateral direction and ventral posterior blade deflection.
Retroperitoneal fat has the tendency to creep into the surgical corridor when the retractor is docked and maximally opened. This may result in injury to surrounding structures. In order to prevent this, we cut the middle 3 fingers off a number 9-sized glove and placed them over the retractor blades (Fig. 2). This provided a barrier from unwanted structures entering between the retractor blades during disc work. We believe that this is a technique modification that may be suited for LLIFs performed in either the prone or lateral decubitus position. Care must be taken that all the rubber is retrieved when removing the retractor. We have, to date, not lost a piece of glove in the retroperitoneum.
Complications
A review of our complications demonstrated a profile similar to LLIF performed in the lateral decubitus position [14]. Complications that have a higher likelihood seen in the prone position due to retractor instability are ALL ruptures and vessel injuries. In our series, we identified 3 ALL ruptures that were treated by redocking the retractor more posteriorly to provide access for thorough posterior disc preparation and more posterior implant placement followed by lateral plate fixation. We then further avoided these injuries by the routine placement of the ALL retractor. We were able to reproducibly appreciate the anterior margin of the disc space and reproducibly work posterior to the anterior annulus. We have found that once placed, the ALL retractor will not migrate ventral or dorsal. This makes it the lighthouse of the approach. Further technologies to improve retractor stability may forgo the routine use of an ALL retractor. Until retractor technologies improve, we recommend the use of an ALL retractor.
Bowel injuries are a rare complication when performing an LLIF [14]. Our team was able to avoid bowel injuries by taking extra time to adequately sweep the retroperitoneal space of all adhesions and placing the dilator over the palmar surface of the hand with a finger touching the psoas. This creates adequate space for safe access through the retroperitoneal space.
Conclusion
Our single-surgeon experience demonstrates the initial learning curve when adopting pLLIF. Thereafter, we experienced reproducibility in our technique and large improvements in our operative times, and complication profile. We found that full access to the anterior and posterior columns gained by prone positioning was beneficial when performing a LLIF. Further studies will include long-term clinical and radiographic outcomes to understand the complete utility of this approach.
References
Ozgur BM, Aryan HE, Pimenta L (2006) Taylor wr extreme lateral interbody fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J 6(4):435–43. https://doi.org/10.1016/j.spinee.2005.08.012
Cappuccino A, Cornwall GB, Turner AW, Fogel GR, Duong HT, Kim KD, Brodke DS (2010) Biomechanical analysis and review of lateral lumbar fusion constructs. Spine 35(26 Suppl):S361-7. https://doi.org/10.1097/BRS.0b013e318202308b
Oliveira L, Marchi L, Coutinho E, Pimenta L (2010) A radiographic assessment of the ability of the extreme lateral interbody fusion procedure to indirectly decompress the neural elements. Spine 35(26 Suppl):S331-7. https://doi.org/10.1097/BRS.0b013e3182022db0
Youssef JA, McAfee PC, Patty CA et al (2010) Minimally invasive surgery: lateral approach interbody fusion: results and review. Spine 35(26):S302–S311
Hijji FY, Narain AS, Bohl DD, Ahn J, Long WW, DiBattista JV, Kudaravalli KT, Singh K (2017) Lateral lumbar interbody fusion: a systematic review of complication rates. Spine J 17(10):1412–1419. https://doi.org/10.1016/j.spinee.2017.04.022
Blizzard DJ, Thomas JA (2018) MIS single-position lateral and oblique lateral lumbar interbody fusion and bilateral pedicle screw fixation: feasibility and perioperative results. Spine 43(6):440–446. https://doi.org/10.1097/BRS.0000000000002330
Sinkov V, Lockey SD, Cunningham BW (2022) Single position lateral lumbar interbody fusion with posterior instrumentation utilizing computer navigation and robotic assistance: retrospective case review and surgical technique considerations. Global Spine J 12(2_suppl):75S-81S. https://doi.org/10.1177/21925682221083909
Nayak AN, Gutierrez S, Billys JB, Santoni BG, Castellvi AE (2013) Biomechanics of lateral plate and pedicle screw constructs in lumbar spines instrumented at two levels with laterally placed interbody cages. Spine J 13(10):1331–1338. https://doi.org/10.1016/j.spinee.2013.03.048
Alluri R, Clark N, Sheha E, Shafi K, Geiselmann M, Kim HJ, Qureshi S, Dowdell J (2021) Location of the femoral nerve in the lateral decubitus versus prone position. Global Spine J. https://doi.org/10.1177/21925682211049170
Lamartina C, Berjano P (2020) Prone single-position extreme lateral interbody fusion (Pro-XLIF): preliminary results. Eur Spine J 29(Suppl 1):6–13. https://doi.org/10.1007/s00586-020-06303-z
Smith TG, Joseph SA Jr, Ditty B, Amaral R, Tohmeh A, Taylor WR, Pimenta L (2021) Initial multi-centre clinical experience with prone transpsoas lateral interbody fusion: feasibility, perioperative outcomes, and lessons learned. N Am Spine Soc J 6:100056. https://doi.org/10.1016/j.xnsj.2021.100056
Walker CT, Farber SH, Gandhi S, Godzik J, Turner JD, Uribe JS (2021) Single-position prone lateral interbody fusion improves segmental lordosis in lumbar spondylolisthesis. World Neurosurg 151:e786–e792. https://doi.org/10.1016/j.wneu.2021.04.128
Rothenfluh DA, Mueller DA, Rothenfluh E, Min K (2015) Pelvic incidence-lumbar lordosis mismatch predisposes to adjacent segment disease after lumbar spinal fusion. Eur Spine J 24(6):1251–1258. https://doi.org/10.1007/s00586-014-3454-0
Uribe JS, Deukmedjian AR (2015) Visceral, vascular, and wound complications following over 13,000 lateral interbody fusions: a survey study and literature review. Eur Spine J 24(Suppl 3):386–396. https://doi.org/10.1007/s00586-015-3806-4
Regev GJ, Chen L, Dhawan M, Lee YP, Garfin SR, Kim CW (2009) Morphometric analysis of the ventral nerve roots and retroperitoneal vessels with respect to the minimally invasive lateral approach in normal and deformed spines. Spine 34(12):1330–5. https://doi.org/10.1097/BRS.0b013e3181a029e1
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Patel, A., Rogers, M. & Michna, R. A retrospective review of single-position prone lateral lumbar interbody fusion cases: early learning curve and perioperative outcomes. Eur Spine J 32, 1992–2002 (2023). https://doi.org/10.1007/s00586-023-07689-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-023-07689-2