Abstract
Purpose
This study aimed to clarify the order of the lumbar maturity stage, each at L1 to L5, and the relationships between age at peak height velocity (APHV) and the lumbar maturity stage.
Methods
A total of 120 male first-grade junior high school soccer players were enrolled and followed for two years, and measurements were performed five times (T1 to T5). The lumbar maturity stage was assessed according to the degree of lesion of the epiphyseal from L1 to L5 using magnetic resonance imaging and classified into three stages: cartilaginous stage, apophyseal stage, and epiphyseal stage. The relationships between T1 and T5 temporal changes and developmental stages divided by 0.5 year increments based on APHV and the lumbar maturity stage at L1 to L5 were examined. For the apophyseal stage, developmental age calculated based on the difference between APHV and chronological age between each lumbar vertebra was compared.
Results
We found that part of the cartilaginous stages decreased as time progressed, while that of the apophyseal and epiphyseal stages increased at L1 to L5 (chi-square test, p < 0.01). L5 matured earlier with the apophyseal stage than L1 to L4 (p < 0.05). The lumbar maturity stage was attained toward L1 from L5, comparing different lumbar levels.
Conclusion
The lumbar maturity stage progresses from L5 toward L1, and the apophyseal and epiphyseal stages would replace the cartilaginous stage at approximately 14 years of age or after APHV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lumbar injuries occur frequently as sports-related injuries during adolescence. Lumbar stress injury and spondylolysis are among the most common lumbar injuries that occur during the adolescent growth spurt, usually among 13–15 year-old individuals. These lumbar injuries are caused by chronic microtrauma or repetitive loading applied to the pars interarticularis in the lumbar spine at an immature and vulnerable stage [1]. The incidence of spondylolysis is estimated to be 3–10% in the general population [2, 3]. Although a previous study reported a 5.9% incidence of lumbar spondylolysis in the general Japanese population [4], Rossi et al. [5] documented a 2–5 times higher incidence in adolescent athletes compared with nonathletes. Once affected, sports activities may have to be restricted for a prolonged period, potentially reducing performance levels after adolescence. Although more knowledge about prevention strategies for lumbar stress injury and spondylolysis is needed, there is currently limited relevant evidence.
Osteochondral injuries, including lumbar spondylolysis, are specific injuries that occur during growth before completion of bone maturation [6, 7]. Because there are differences in the completion rate of bone maturation depending on the body part, understanding the period of bone maturation is important, especially for body parts more prone to sports injuries. Ikata et al. [8] assessed the maturity stage of the lumbar vertebral body according to the degree of lesion of the epiphyseal and classified it into three stages: cartilaginous, apophyseal, and epiphyseal stages. Uraoka et al. [9] examined age-related changes in lumbar maturation stages at L3 in patients aged 10–18 years and found that the apophyseal stage peaked at the age of 14 years. Because the optimal age for lumbar stress injury and spondylolysis corresponds to the secondary ossification stage, i.e., the apophyseal stage, it is suggested that there is a relationship between the lumbar maturity stage and the occurrence of injury. However, further investigation is necessary considering the following points: (1) it is unclear whether the maturity of L1 to L5 is the same as that of L3 and (2) it is insufficient to evaluate the lumbar maturity stage during adolescence based on chronological age alone, owing to vast variations in biological maturity among individuals.
Therefore, this study aimed to clarify (1) the order of the lumbar maturity stage, each of L1 to L5, during adolescence and (2) the relationships between biological maturation based on the age at peak height velocity (APHV) and the lumbar maturity stage. We hypothesized that lumbar maturation may occur from L1 or L5, as Rauch et al. [10] reported that limb growth precedes central relative to peripheral.
Methods
Participants
A total of 120 male first-grade junior high school soccer players were enrolled in this study and followed for 2 years. There were five measurement timings: first year middle school, April–May (T1); first year middle school, October–November (T2); second year middle school, April–May (T3); second year middle school, October–November (T4); and first year middle school, April–May (T5). The team was part of a local recreational league, and the participants attended regular football training after school and on the weekends. No athletes had disorders or illnesses that could affect their daily life or physical growth, such as idiopathic scoliosis or invasive surgical treatments.
All measurements were performed at our institution. This study was approved by the ethics committee of the XXXXXX, XXXXXX and the participants and their parents provided signed informed consent before participation.
Lumbar maturity stage assessment
To evaluate the lumbar maturity stage, the players were examined using a 1.5-Tesla whole-body magnetic resonance imaging (MRI) system (Signa 1.5T; GE Healthcare, Waukesha, WI) and evaluated based on short-time inversion recovery images (echo time: 47.4 ms; repetition time: 3000 ms; slice thickness: 4 nm). The lumbar maturity stage was assessed based on the degree of lesion of the epiphyseal from L1 to L5 as described by Ikata et al. [8] and classified into three stages: cartilaginous, apophyseal, and epiphyseal stages. The evaluations regarding the lumbar maturity stages of all participants were performed by an experienced orthopedic surgeon (Su.T.). One of the co-authors, also an orthopedic surgeon (Se. T.), assessed the lumbar maturity stages at a total of 100 vertebral levels, including L1 through L5 in 20 participants, to confirm inter-rater reliability. The images used to evaluate the reliability of the lumbar maturity stages were provided by one author (T.T.), so that the two examiners' assessments were blinded to each other. Thus, an almost perfect agreement between the observers was found, with a k value of 0.86 (95% confidence interval: 0.79–0.93). The determination of all lumbar maturity stages was performed in an anonymized setting by a third party to ensure that the results were blinded.
Developmental age
APHV, a well-established event of an adolescent growth spurt, was evaluated using the AUXAL 3.1 program (Scientific Software International Inc., Skokie, IL, USA), using their height records of the past 6 years and their current height measured in this study. Data on their heights measured annually at school during elementary schooling were collected from their parents. We calculated the developmental age of each participant by subtracting their chronological age from their APHV to determine the period until their peak of growth. In other words, the current time is before APHV if developmental age is negative, and APHV has already passed if developmental age is positive.
Statistical analysis
SPSS Statistics 27 (SPSS, Inc., IBM, Japan) was used to analyze the dataset. The five timing (T1–T5) changes in the lumbar maturity stages (cartilaginous, apophyseal, and epiphyseal stages) in each lumbar spine were analyzed using the chi-square test. We used two methods to compare the relationships between the lumbar maturity stages and developmental age. First, we categorized the developmental age into eight developmental stages in increments of 0.5 years and examined the differences in the lumbar maturity stage based on developmental stage using the chi-square test as mentioned above. Second, differences in developmental age according to the lumbar level (L1–L5) were analyzed using one-way analysis of variance (post hoc: Tukey). Because the age group in this study was adolescents aged 12–15 years, and the proportion of apophyseal stages was high, we compared only apophyseal stages in terms of developmental age [9]. Statistical significance was set at P value < 0.05.
Results
Table 1 shows the age, height, weight, and developmental age from T1 to T5. The mean APHV of all participants was 13.3 ± 0.8 years. When changes in the lumbar maturity stage were examined for each lumbar level over time, significant differences were found at all lumbar levels and timings except for T3–T4 at L4 (Fig. 1). At all lumbar levels, the proportion of cartilaginous stages decreased as time progressed, whereas the proportion of apophyseal and epiphyseal stages increased.
Changes in the lumbar maturity stage over time from L1 to L5. Asterisk (*) indicates significant differences from the previous time point. C, Cartilaginous stage; A, Apophyseal stage; E, Epiphyseal stage; T1, April–May in the first year of junior high school; T2, October–November in the first year of junior high school; T3, April–May in the second year of junior high school; T4, October–November in the second year of junior high school; T5, April–May in the first year of junior high school
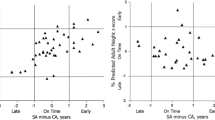
Table 2 shows the classification of developmental stages and the characteristics of the participants in each category. In the first analysis, i.e., categorizing developmental age into eight developmental stages in increments of 0.5 years, the difference of proportion in the three lumbar maturity stages according to developmental stage was significant (chi-square test, p < 0.01), and changes in lumbar maturity were observed especially from developmental stages 3 to 6 at all lumbar levels (Fig. 2). The lumbar maturity stage matured toward L1 from L5 with respect to the different lumbar levels.
The second analysis compared developmental age across lumbar levels in the apophyseal stage (Fig. 3) and revealed that L5 matured earlier than L1 (p < 0.001), L2 (p < 0.001), L3 (p < 0.001), and L4 (p = 0.012), while L4 matured earlier than L1 (p < 0.001) and L2 (p = 0.005).
Discussion
Our study identified 1) the order of the lumbar maturity stage each of L1 to L5 during adolescence and (2) the relationships between biological maturation based on the APHV and the lumbar maturity stage to provide useful knowledge that could contribute to the prevention and treatment of lumbar injuries that frequently occur during adolescence. Because it was previously known that the lumbar maturity stage changes significantly around the age of 13–14 years [9], we investigated the maturation order during adolescence in detail. The main findings were the transition from the cartilaginous stage to the apophyseal and epiphyseal stages during adolescence and that the lower lumbar vertebrae mature earlier. In addition, the results of change in the percentage of the lumbar maturity stage over time also showed that lumbar maturation progresses during adolescence, even if only in 6 months.
We found the lumbar maturation stage to progress from L5 to L1 and the apophyseal stage to change to the epiphyseal stage after T5 (14.60 ± 0.03 years) or developmental stage 7 (after 1 year of APHV) for L5. A previous study reported the appearance of secondary ossification of the sacrum adjacent to the lumbar spine after the age of 12 years [11] as well as at 11–13 years for the iliac crest [12, 13] and 13–15 years for the ischium [14], which compose the pelvic girdle. Ossification centers begin to fuse first in the cervical and lumbar vertebrae, followed by the thoracic region. Given the relationship between age and the timing of fusion of ossification centers, the spine would mature from the peripheral (caudal or head side) to the central area.
This is the first study to investigate the relationships between biological maturation indicated by developmental age and the lumbar maturity stage. One of the most accurate methods for evaluating biological maturity has known to be skeletal age based on bone maturation. On the other hand, using X-rays makes it unsuitable for application in sports settings due to concerns regarding time, cost, and radiation exposure. Several studies have examined the relationship between developmental age assessed based on APHV and biological maturity status indicated by skeletal age and have reported a correlation between them [15]. Considering that the developmental stage, maturation period, and growth rate at a particular time differ among individuals during the growth spurt, the chronological age index alone is insufficient. Therefore, the developmental age-based evaluation used in this study is considered an extremely accurate assessment of changes in the lumbar maturity stage during an adolescent growth spurt. In addition, the APHV obtained as the standard for maturity status was 13.3 ± 0.8 years, which is close to what has been reported previously for the Japanese population [16]. The apophyseal stage at L5 was found to correspond approximately to APHV. Charles et al. [17] suggested using the olecranon apophyseal fusion sequence alone to determine the PHV in patients at Risser grade 0. Based on the results of this study, it might be possible to speculate on APHV based on the lumbar maturation stage at L5 in clinical facilities where X-rays and MRIs are available.
Osteochondral injuries occurring during the growth spurt are thought to be caused by repetitive local stress through sports activities while the bones are still immature [6, 7]. Lumbar spondylolysis is one of the major growth-phase-specific sports injuries that occur in the lumbar spine and is believed to be caused by frequent mechanical loading of the pars interarticularis during the period of lumbar immaturity [18]. The prevalence of lumbar spondylolysis in youth soccer players is high [19]. Because the participants in our study were also soccer players, recognizing the growth pattern of lumbar maturity stages may help identify players at risk of lumbar spondylolysis. A previous study has shown that lumbar spondylolysis occurs more frequently at L5 [20]. The factors and mechanisms are under investigation, but the phenomenon may be related to the growth pattern of the lumbar spine. Osgood’s disease, a growth-specific injury common to lumbar spondylolysis, is considered to be at risk due to the apophyseal stage of tibial tuberosity [21]. Saver’s disease has also been shown to be more likely to occur at stage 2 (stages are indicated from 0 to 5), defined as when the apophysis covers > 50% of the metaphysis but does not extend to the plantar edge [22]. Kaneko et al. [23] reported that the incidence of lumbar stress injury at L5, which is considered a precursor to lumbar spondylolysis, was > 30% at the age of ≥ 13 years. This corresponded to the age older than T2 (12.98 ± 0.03 years) in our study.
Given that the lumbar maturity stage progresses from L5 to L1 and that the proportion of the cartilaginous stage of lumbar maturity, which has not completed maturation, was high in T1 and T2, the transition from cartilaginous to the apophyseal stage may be one of the risk factors for lumbar spondylolysis. Sairyo et al. [24] found that the immature cartilaginous stage was a risk factor for further slippage. Based on this report, more attention is required for athletes before T3 or APHV when a high proportion of the cartilaginous stage is present if they have complaints of lumbar disorders, including low back pain.
Our study has several limitations. First, MRI was performed every 6 months, so it is impossible to determine which lumbar maturity stage was assessed during the cartilaginous, apophyseal, and epiphyseal stages. Second, although APHV in our study was shown as a normative value in the Japanese population, the possibility of selection bias cannot be ruled out.
In conclusions, it is revealed that the lumbar maturity stage progresses from L5 toward L1, and the apophyseal and epiphyseal stages would replace the cartilaginous stage at approximately 14 years of age or after APHV.
Data availability
Datasets generated and/or analyzed during the current study are not publicly available due to the wishes of the participant's team director, but are available from the corresponding author upon reasonable request.
References
Leone A, Cianfoni A, Cerase A et al (2011) Lumbar spondylolysis: a review. Skeletal Radiol 40:683–700. https://doi.org/10.1007/s00256-010-0942-0
Beutler WJ, Fredrickson BE, Murtland A et al (2003) The natural history of spondylolysis and spondylolisthesis: 45-year follow-up evaluation. Spine 28:1027–1035. https://doi.org/10.1097/01.BRS.0000061992.98108.A0
Sonne-Holm S, Jacobsen S, Rovsing HC et al (2007) Lumbar spondylolysis: a life long dynamic condition? A cross sectional survey of 4.151 adults. Eur Spine J 16:821–828. https://doi.org/10.1007/s00586-006-0250-5
Sakai T, Sairyo K, Suzue N et al (2010) Incidence and etiology of lumbar spondylolysis: review of the literature. J Orthop Sci 15:281–288. https://doi.org/10.1007/s00776-010-1454-4
Rossi F, Dragoni S (2001) The prevalence of spondylolysis and spondylolisthesis in symptomatic elite athletes: radiographic findings. Radiography 7:37–42. https://doi.org/10.1053/radi.2000.0299
McKay D, Broderick C, Steinbeck K (2016) The adolescent athlete: a developmental approach to injury risk. Pediatr Exerc Sci 28:488–500. https://doi.org/10.1123/pes.2016-0021
Gerrard DF (1993) Overuse injury and growing bones: the young athlete at risk. Br J Sports Med 27:14–18. https://doi.org/10.1136/bjsm.27.1.14
Ikata T, Miyake R, Katoh S et al (1996) Pathogenesis of Sports-related spondylolisthesis in adolescents: radiographic and magnetic resonance imaging study. Am J Sports Med 24:94–98. https://doi.org/10.1177/036354659602400117
Uraoka H, Higashino K, Morimoto M et al (2018) Study of lesions of the lumbar endplate based on the stage of maturation of the lumbar vertebral body: the relationship between skeletal maturity and chronological age. Eur J Orthop Surg Traumatol 28:183–187. https://doi.org/10.1007/s00590-017-2032-7
Rauch F, Bailey DA, Baxter-Jones A et al (2004) The ‘muscle-bone unit’ during the pubertal growth spurt. Bone 34:771–775. https://doi.org/10.1016/j.bone.2004.01.022
Götz W, Funke M, Fischer G et al (1993) Epiphysial ossification centres in iliosacral joints: anatomy and computed tomography. Surg Radiol Anat 15:131–137. https://doi.org/10.1007/BF01628312
Zejden A, Jurik AG (2017) Anatomy of the sacroiliac joints in children and adolescents by computed tomography. Pediatr Rheumatol 15:82. https://doi.org/10.1186/s12969-017-0210-0
Grissom LE, Harty MP, Guo GW, Kecskemethy HH (2018) Maturation of pelvic ossification centers on computed tomography in normal children. Pediatr Radiol 48:1902–1914. https://doi.org/10.1007/s00247-018-4233-6
Eich GF, Babyn P, Giedion A (1992) Pediatric pelvis: radiographic appearance in various congenital disorders. Radiographics 12:467–484. https://doi.org/10.1148/radiographics.12.3.1609139
Malina RM, Dompier TP, Powell JW et al (2007) Validation of a noninvasive maturity estimate relative to skeletal age in youth football players. Clin J Sport Med 17:362–368. https://doi.org/10.1097/JSM.0b013e31815400f4
Takei S, Taketomi S, Tanaka S, Torii S (2019) Growth pattern of lumbar bone mineral content and trunk muscles in adolescent male soccer players. J Bone Miner Metab 38:1–8
Charles YP, Diméglio A, Canavese F, Daures JP (2007) Skeletal age assessment from the olecranon for idiopathic scoliosis at risser grade 0. J Bone Jt Surg-Am Vol 89:2737–2744
Sairyo K, Goel VK, Masuda A et al (2006) Three dimensional finite element analysis of the pediatric lumbar spine. Part II: biomechanical change as the initiating factor for pediatric isthmic spondylolisthesis at the growth plate. Eur Spine J 15:930–935. https://doi.org/10.1007/s00586-005-1033-0
Tatsumura M, Gamada H, Ishimoto R et al (2018) Prevalence of curable and pseudoarthrosis stages of adolescent lumbar spondylolysis. J Rural Med 13:105–109. https://doi.org/10.2185/jrm.2967
Grogan JP, Hemminghytt S, Williams AL et al (1982) Spondylolysis studied with computed tomography. Radiology 145:737–742. https://doi.org/10.1148/radiology.145.3.7146406
Kaneuchi Y, Otoshi K, Hakozaki M et al (2018) Bony maturity of the tibial tuberosity with regard to age and sex and its relationship to pathogenesis of osgood-schlatter disease: an ultrasonographic study. Orthop J Sports Med 6:232596711774918. https://doi.org/10.1177/2325967117749184
Duong MM, Nicholson AD, Li SQ et al (2020) Relationship between sever disease and skeletal maturity. J Pediatr Orthop 40:93–96. https://doi.org/10.1097/BPO.0000000000001145
Kaneko H, Murakami M, Nishizawa K (2017) Prevalence and clinical features of sports-related lumbosacral stress injuries in the young. Arch Orthop Trauma Surg 137:685–691. https://doi.org/10.1007/s00402-017-2686-y
Sairyo K, Katoh S, Ikata T et al (2001) Development of spondylolytic olisthesis in adolescents. Spine J 1:171–175. https://doi.org/10.1016/S1529-9430(01)00018-3
Acknowledgements
The authors sincerely thank the players who participated in this study. We also thank Nao Shinoda, the head coach of the junior soccer team in Tokyo.
Funding
No funds, Grants, or other support was received. The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by TT, ST and ST. The first draft of the manuscript was written by TT and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Consent to participate
All participants and their parents provided signed informed consent for participating in the study.
Consent for publication
Informed consent was obtained from all individual participants and their parents included in the study.
Ethics approval
This study was approved by the ethics committee of Waseda University (approval number: 2021-218).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tsutsui, T., Iizuka, S., Takei, S. et al. Growth pattern of lumbar maturity stage at L1 to L5 during adolescent growth spurt. Eur Spine J 32, 2164–2170 (2023). https://doi.org/10.1007/s00586-023-07686-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-023-07686-5