Abstract
Study Design
Prospective observational cohort study.
Objective
To understand if serum procalcitonin (PCT) is a reliable indicator of sepsis in spinal cord injury (SCI) patients for better prognosis and earlier diagnosis when compared with other common biomarkers such as C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), white blood cells (WBC), blood culture and body temperature.
Methods
From March 2021 to August 2022, data were collected for SCI patients who developed septicaemia. In addition to neurology and admission, the following blood samples were collected on day one of infection: PCT, CRP and WBC. Linear regression analysis was performed to determine the relationship between PCT, CRP and WBC.
Results
A total of 27 SCI patients had an infection during their stay in the regional centre; however, only 10 developed septicaemias. 100% of SCI individuals with sepsis had elevated PCT levels, whilst 60% had elevated CRP and 30% had elevated WBC levels. There was a strong positive correlation between PCT and CRP (R2 = 0.673, CI = 95%, 5.5–22.8, p < 0.05) and a weaker positive correlation between PCT and WBC (R2 = 0.110, CI = 95%, 4.2–10.9, p < 0.05).
Conclusion
In SCI individuals, there was a correlation between serum PCT levels and septicaemia. Alongside this, PCT appeared to be more consistent throughout the study population when compared with CRP and WBC. However, this was a preliminary study and further research is required on a larger scale.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sepsis is the body’s overactive and extreme response to an infection—a life-threatening medical emergency which can lead to organ failure (severe sepsis) and death if left untreated. Sepsis is defined as confirmed or suspected infection associated with a systemic inflammatory response syndrome (SIRS). This infection can also lead to septic shock—which leads to an exaggeration of persistent hypotension within the spinal cord injury population [1].
Urinary tract and chest infections leading to sepsis are major cause of mortality and morbidity in critically ill spinal cord injury (SCI) people. According to a comprehensive review by McCaughey et al., the United Kingdom (UK) experiences around 16 new cases per million population in traumatic spinal cord injuries and 2–3 new cases per million population in non-traumatic spinal cord injuries per year. [2] The potential for sepsis in individuals with a SCI is higher when compared to an able-bodied person. For those with paralysis, the common causes of sepsis result from infected pressure sores and urinary tract infections (urosepsis). Respiratory tract infections (such as pneumonia) are common during initial admission with a reported frequency of up to 60%, with urinary tract infections and pressure ulcers having incidences of 34% and 10%, respectively. [3, 3]
In this study, we investigated the response of procalcitonin (PCT) levels in serum blood of SCI patients who encountered septicaemia post-injury. Subsequently, we compared the change of PCT to common infective biomarkers such as white blood cell count (WBC), C-reactive protein (CRP), body temperature and blood cultures.
The reason for this investigation is to compare SCI persons to an able-bodied person to understand if serum PCT is as reliable of an indicator as it has proven to be in able-bodied individuals. SCI is a high disabling injury and results in damage or loss of sensational and motor function—including vascular ischaemia. In able-bodied individuals, heat loss is mainly regulated by sweating. A tetraplegic individual (e.g. as a result of cervical cord transection) is unable to sweat and it has been suggested that a supplementary hear loss mechanism is an amplification of the ventilatory response [5]. This implies that general normal functions are altered and responses are more complex. Tetraplegic people are known to interrupt sympathetic vasculature control. This prevents shunting of blood from the periphery to central organs when exposed to colder temperatures, leading them to risk of hypothermia. The interruption of sensory pathways to the cortex and hypothalamus impairs behavioural and involuntary responses to cold ambient temperatures [6].
Patients and methods
Data collection
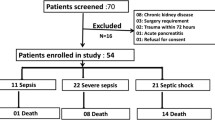
This prospective observational cohort study was conducted in a regional care centre—which overlooks the region of more than 5,000,000 people. The study took place from March 2021 up until August 2022, and data were collected from all in-patients aged above 18 with a SCI and whether they encountered an infection throughout their admission. A total of 27 patients matched the criteria for the study. Clinically relevant data were collected from all these patients including age, gender, initial diagnosis, type of neurology and date of infection with the cause (if an infection was present).
From these patients, we obtained blood samples if they exhibited signs and symptoms of septicaemia whilst present in the ward based on the NICE Guidelines. Throughout the 17 months, 10 individuals suffered a complication leading to septicaemia. 17 people were excluded from the study population as they were not septic individuals and suffered infections from other causes (non-infectious or COVID). The initial date of infection alongside the cause was recorded—which were urosepsis (via a urinary tract infection), chest infection or infection via a sacral pressure sore. On day one of infection, we collected relevant parameters via blood tests including PCT, CRP, WBC count, blood cultures and body temperature.
Statistics
Statistical analysis of this data was then performed using Statistical Package for Social Survey (SPSS) Statistics (version 28.0.1.1 (14), SPSS Inc., Chicago, IL). The data were analysed using a statistical significance of p < 0.05 for all comparisons with a confidence interval of 95%. The results were also tabulated and graphically represented using SPSS Statistics. The quantitative variables are expressed as mean and one standard deviation, and the qualitative variables are expressed as frequencies and percentages.
Results
Inclusion and exclusion criteria
Overall, 10 patients were included in the study from a total of 27 screened for the inclusion and exclusion criteria during the study period. Demographics of the 27 patients can be found in (Table 1) which mentions the gender, mean age (±standard deviation), age range, age groups (adults or seniors), type of neurology (paraplegic or tetraplegic) and reason for admission (traumatic or non-traumatic injury).
The reason for exclusion of 17 patients from the study population can be found in (Table 2). This shows that 11 patients had infections due to COVID and 6 were non-infectious throughout their admission. From the remaining 10 patients, 7 had a urinary tract infection (UTI), 2 had a chest infection and 1 had an infection of a sacral pressure sore leading to a total of 10 septic patients. These 10 patients had their PCT levels measured—the demographics of these were a mean age of 60.50 (±16.74) and an age range of 28–82 which was very similar to the initial population.
Blood serum results
The results from the blood samples of the 10 septic patients, taken on day one of infection, can be seen in (Table 3). The normal ranges for PCT, CRP, WBC and body temperature were taken from the local trust reference ranges.
All people with sepsis had elevated PCT levels. 60% had elevated CRP levels with 40% having normal values and 30% had elevated WBCC levels with 70% having a normal WBCC. Only 2 cases of positive blood cultures were found—one strain being Staphylococcus aureus and the other being Staphylococcus ominis. 80% of patients had an increased body temperature (> 37.5 °C) with 20% being within the normal range of 36.0–37.5 °C. This signifies that the majority of individuals with elevated PCT levels and septic symptoms had negative blood cultures and spiked body temperatures.
Blood serum biomarker relationships
The relationship between CRP and PCT can be shown in (Fig. 1A). As a general trend, as CRP values increased, the value of PCT also increased—showing positive correlation. The confidence intervals were 95%, 5.5 to 22.8, with p < 0.05 and there was a weak strong correlation with an R2 value of 0.673.
Figure 1B also shows the relationship between WBC and PCT. This implies a much weaker correlation between the two variables with an R2 value of 0.110; however, the general trend was as WBC increased, PCT values also increased—a weaker positive correlation. This graph was also analysed with 95% confidence intervals, 4.2 to 10.9, with p < 0.05.
We compared whether the type of neurology and the infection leading to the septicaemia influenced the PCT values. Initially, the type of neurology had no significant difference on the mean PCT value as both paraplegic and tetraplegic individuals averaged a mean PCT of 1.00 ng/ml. This can be viewed in (Fig. 2). Furthermore, urosepsis had shown to cause a significantly higher mean PCT compared to chest infection and sacral pressure sore infections.
Discussion
The main findings of our study concluded that out of the entire study population, the PCT values were all elevated in SCI patients with sepsis. CRP values were also raised in 80% of the population whereas WBC were only elevated in 30% of the population. Additionally, only 80% of the population had a raised body temperature.
In able-bodied individuals, CRP is known to be highly suggestive of sepsis (sensitivity 98.5%, specificity 75%) and in individuals with severe sepsis, hypothermia (≤ 36.5 °C) was associated with increased mortality and organ failure, irrespective of the presence of septic shock [7, 8].
This further signifies that general normal functions are altered, and responses are more complex in SCI individuals. This was concluded from data suggesting individuals with tetraplegia after SCI have significant dysfunction of thermoregulation associated with frequent episodes of subnormal body temperature in a normal ambient environment—with 66% having subnormal body temperatures [6]. However, our study concluded that the majority of patients faced a spike in body temperature (hyperthermia) on day one of sepsis.
Alongside hypothermia, individuals with SCI are prone to bradycardia and hypotension (nephrogenic shock and orthostatic hypotension). These are alongside further complications such as pain, spasticity and autonomic dysreflexia [9].
Septic shock presents with signs such as hypothermia, hypotension and tachycardia for an able-bodied person. However, as aforementioned, SCI individuals present with these signs pre-sepsis. Therefore, our aim is to identify whether PCT is a suitable marker for early diagnosis of sepsis.
The main complications in SCI people leading to sepsis are pressure sores and bladder infections. These are common complications and adequate prevention requires identifying the risk at an early stage. Pressure sores are the most common chronic complication [10].
Individuals with a SCI are at risk of developing pressure sores because they sit for prolonged periods of time and have impaired mobility with a loss of sensation. The prevalence of pressure sores varies between 9.6% and 47.4% in individuals with SCI. Pressure sores are an important and potentially life-threatening secondary complication of SCI. They can lead to further functional disability and fatal infections and surgical interventions can be required [11]. Diseases of skin (including pressure sores) were reported as the second most common aetiology for rehospitalisation at most time intervals (years 1, 10, 15, 20) in a multicentre analysis with SCI individuals [3].
Bladder distension is the most common triggering factor for autonomic dysreflexia (AD). The distension can result from urinary retention or catheter blockage and accounts for up to 85% of cases [12].
Indwelling urethral catheters are used in the acute phase of injury but they are not recommended for long-term use because of the high risk for urinary complications (e.g. urinary tract infection, calculi, urethral damage, renal dysfunction and bladder cancer). Singh et al. identified an indwelling catheter as being the most prevalent risk indicator of urinary tract infection in SCI individuals. The risk of urinary tract infection increases with the increasing duration of catheterization [13].
With early detection of infection leading to better prognosis, PCT and CRP are the most frequently used biomarkers in sepsis across all patient types [14].
Procalcitonin is a biological marker in the blood which has been found to increase during a bloodstream infection. A high-level correlates to a sign of a serious bacterial infection. If treated, the decreasing level can reflect the effect of the intervention. Furthermore, serum PCT evaluation has been proposed for early diagnosis and accurate staging to guide decisions regarding individuals with sepsis, severe sepsis and septic shock [15].
Accordingly, high early levels of PCT in sepsis have been suggested to be associated with an unfavourable prognosis. The measurement of PCT has gained interest due to it being a quantitative test that exhibits predictable values and is more responsive to post-operative events as compared with more common infection measurements such as WBC, CRP and erythrocyte sedimentation rate (ESR). It has been largely confirmed that PCT is the only biochemical parameters among a large array which closely correlates with the inflammatory host response to microbial infections. One study found that PCT was an excellent early predictor or diagnostic parameter, superior to CRP [16].
This was further supported in a recent study where PCT was superior to CRP in differentiating bacterial causes of inflammation from non-infectious causes [17].
Spinal cord injury individuals are a unique set of individuals—with multi-system involvement and autonomic dysfunction leading to abnormal reactions to many secondary complications. Our aim is to conclude whether the policy regarding measuring PCT in able-bodied individuals for sepsis is also appropriate and required in SCI individuals.
In SCI individuals, checking PCT levels is still not a requirement when investigating for septic patients. It has been vastly concluded that PCT is one of the only blood serum parameters which closely correlates with bacterial infections. Serum PCT evaluation was proposed for early diagnosis and accurate staging of sepsis, contributing to early decisions and optimal care for the able-bodied person—leading to better outcomes in individuals with sepsis, severe sepsis and septic shock [18]. Having systematically searched through many databases including PubMed, Cochrane, Embase and MEDLINE, we were unable to find a study to discern if PCT is a reliable indicator of sepsis in SCI patients comparatively to CRP, ESR, WBC and other biochemical markers. This was the basis for the commencement of this study and thus has allowed us to demonstrate that PCT does increase in SCI individuals with sepsis. We would recommend further studies to be undertaken to supplement further findings to support our study.
This study will be useful in the clinical setting by allowing clinicians to consider another infection marker for treatment. As well as treating sepsis using PCT as a marker, antibiotic stewardship is becoming more of a prevalent issue which also needs to be addressed. The increased use of antibiotics is leading to the emergence of antibiotic resistance; therefore, targeted prescribing is essential as well as improving knowledge about antibiotic prescribing. [19] Gregoriano et al. [20] discussed the importance of procalcitonin in the management of sepsis. The paper highlighted the major challenge of using antibiotics to treat sepsis and how the use of PCT in managing sepsis is important as it has good discriminatory properties to differentiate between bacterial and viral infections and thus can help improve antibiotic stewardship by reducing the prevalence of antibiotic prescribing in viral infections. In a systematic review of using PCT-guided therapy in intensive care unit patients with severe sepsis and septic shock, it has been concluded that PCT is useful in helping clinicians decide about antibiotic therapy and ensuring there is a shorter duration of antibiotic use compared to standard care [21]. From this study, it can be concluded that PCT is useful for clinicians treating patients as it ensures a targeted approach to treatment, leading to shorter durations of antibiotic use. For SCI patients in particular, the previous studies would help show the benefits with using PCT. SCI patients are particularly vulnerable to infections especially those respiratory in nature. Sepsis occurs as a secondary complication after SCI and has terrible outcomes for patients [22]. Therefore, PCT-guided therapy can help SCI patients suffering from sepsis in an intensive care setting and, however, further studies to understand its effectiveness in that setting.
The limitations we faced throughout this study included being unable to test the ESR values for all patients which hindered our ability to compare how the ESR levels were affected in SCI patients as it is also a key inflammatory biomarker.
Further to issues regarding taking samples, we were unable to take PCT values for non-septic patients due to policies. This meant we were unable to conduct Student’s t tests and ROC curves to test for PCT sensitivity and specificity—as the student’s t test is used to investigate differences between groups depending on the distribution of data—meaning we were unable to see where PCT had a significance depending on sepsis.
Unfortunately, the study population was very limited throughout the study time. A smaller sample size invariably leads to a decrease in reliability. As discussed in Tipton et al. [23], a small sample size can lead to large differences between the sample and population by chance. Therefore, if future studies were to occur, they would require a much larger sample size. This will enable statistics to be developed on a larger population and will increase the statistical power of the study. PCT values would also need to be taken of non-septic infections to provide a control group in order to compare the effect of PCT levels. This will enable a future study to be performed to investigate if PCT increase is significant within the SCI group only. From this, it can help formulate a proforma for investigating and treating sepsis in spinal cord injury patients.
It would be beneficial to monitor the response of PCT throughout the treatment of septicaemia—not only on day one. This was limited in our study due to the lack of emphasis on testing for PCT in SCI patients with sepsis.
Conclusion
PCT levels were elevated in all SCI patients with sepsis in this study, suggesting serum PCT levels are a reliable biological marker for early prediction and diagnosis of septic complications in SCI patients. Other biomarkers including CRP and body temperature were also indicative of inflammation; however, there were cases emphasising abnormal able-bodied reactions to a sepsis infection in SCI individuals. However, due to the size of the study population, sensitivity and specificity were unable to be measured comparing PCT with other biological markers. We would recommend PCT levels to be checked if a person with a SCI was to be infected; however, the results are considered preliminary and further research is required to verify the findings in the form of a large-scale multicentre prospective observational trial with PCT values taken from day 1 of infection and throughout the treatment of the infection. These should also include those with an infection that is not sepsis to be used as a control group in order to compare the significance of PCT to CRP and ESR. This will aid in determining whether testing PCT levels would be a necessity for infections in SCI individuals to diagnose sepsis at an early stage and to implement this in clinical practice and policy.
Data availability
Data are available upon request.
References
Jawad I, Lukšić I, Rafnsson SB (2012) Assessing available information on the burden of sepsis: global estimates of incidence, prevalence, and mortality. J Glob Health. https://doi.org/10.7189/jogh.01.010404
McCaughey EJ, Purcell M, McLean AN, Fraser MH, Bewick A, Borotkanics RJ et al (2016) Changing demographics of spinal cord injury over a 20-year period: a longitudinal population-based study in Scotland. Spinal Cord 54(4):270–276
Cardenas DD, Hoffman JM, Kirshblum S, McKinley W (2004) Etiology and incidence of rehospitalization after traumatic spinal cord injury: a multicenter analysis. Arch Phys Med Rehabil 85(11):1757–1763
Waites KB, Canupp KC, Chen Y, DeVivo MJ, Moser SA (2001) Bacteremia after spinal cord injury in initial versus subsequent hospitalizations. J Spinal Cord Med 24(2):96–100
Silver JR (2007) Thermoregulation in tetraplegic patients. Spinal cord 45(6):460
Khan S, Plummer M, Martinez-Arizala A, Banovac K (2007) Hypothermia in patients with chronic spinal cord injury. J Spinal Cord Med 30(1):27–30
Povoa P, Almeida E, Moreira P, Fernandes A, Mealha R, Aragao A et al (1998) C-reactive protein as an indicator of sepsis. Intensiv Care Med 24(10):1052–1056
Kushimoto S, Gando S, Saitoh D, Mayumi T, Ogura H, Fujishima S et al (2013) The impact of body temperature abnormalities on the disease severity and outcome in patients with severe sepsis: an analysis from a multicenter, prospective survey of severe sepsis. Crit Care 17(6):1–9
Hagen EM (2015) Acute complications of spinal cord injuries. World J Orthop 6(1):17
Alexander MS, Biering-Sorensen F, Bodner D, Brackett NL, Cardenas D, Charlifue S et al (2009) International standards to document remaining autonomic function after spinal cord injury. Spinal cord 47(1):36–43
Regan MA, Teasell RW, Wolfe DL, Keast D, Mortenson WB, Aubut JA et al (2009) A systematic review of therapeutic interventions for pressure ulcers after spinal cord injury. Arch Phys Med Rehabil 90(2):213–231
Shergill IS, Arya M, Hamid R, Khastgir J, Patel HR, Shah PJ (2004) The importance of autonomic dysreflexia to the urologist. BJU Int 93(7):923–926
Singh R, Rohilla RK, Sangwan K, Siwach R, Magu NK, Sangwan SS (2011) Bladder management methods and urological complications in spinal cord injury patients. Indian J Orthop 45(2):141–147
Ryu JA, Yang JH, Lee D, Park CM, Suh GY, Jeon K et al (2015) Clinical usefulness of procalcitonin and C-reactive protein as outcome predictors in critically ill patients with severe sepsis and septic shock. PLoS ONE 10(9):e0138150
Kumar A (2010) Early antimicrobial therapy in severe sepsis and septic shock. Curr Infect Dis Rep 12(5):336–344
Müller F, Christ-Crain M, Bregenzer T, Krause M, Zimmerli W, Mueller B et al (2010) Procalcitonin levels predict bacteremia in patients with community-acquired pneumonia: a prospective cohort trial. Chest 138(1):121–129
Simon L, Saint-Louis P, Amre DK, Lacroix J, Gauvin F (2008) Procalcitonin and C-reactive protein as markers of bacterial infection in critically ill children at onset of systemic inflammatory response syndrome. Pediatr Crit Care Med 9(4):407–413
Kenzaka T, Okayama M, Kuroki S, Fukui M, Yahata S, Hayashi H et al (2012) Use of a semiquantitative procalcitonin kit for evaluating severity and predicting mortality in patients with sepsis. Int J Gen Med 5:483
Johnson AP, Ashiru-Oredope D, Beech E (2015) Antibiotic stewardship initiatives as part of the UK 5-year antimicrobial resistance strategy. Antibiotics (Basel) 4(4):467–479. https://doi.org/10.3390/antibiotics4040467
Gregoriano C, Heilmann E, Molitor A, Schuetz P (2020) Role of procalcitonin use in the management of sepsis. J Thorac Dis 12(Suppl 1):S5–S15. https://doi.org/10.21037/jtd.2019.11.63
Prkno A, Wacker C, Brunkhorst FM, Schlattmann P (2013) Procalcitonin-guided therapy in intensive care unit patients with severe sepsis and septic shock—a systematic review and meta-analysis. Crit Care 17(6):R291. https://doi.org/10.1186/cc13157
Weiterer S, Frick S, Lichtenstern C, Hug A, Uhle F, Weigand MA, Hundt G, Siegler BH (2019) Sepsis in mechanically ventilated patients with spinal cord injury: a retrospective analysis. Spinal Cord 57(4):293–300. https://doi.org/10.1038/s41393-018-0217-5. (Epub 2018 Nov 9)
Tipton E, Hallberg K, Hedges LV, Chan W (2017) Implications of small samples for generalization: adjustments and rules of thumb. Eval Rev 41(5):472–505. https://doi.org/10.1177/0193841X16655665. (Epub 2016 Jul 8)
Acknowledgements
Acknowledgements to the Yorkshire Regional Spinal Injuries Centre.
Funding
No financial assistance was used for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
We certify that all applicable institutional and governmental regulations were followed, and confidentiality was kept throughout the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Anas, M., Hasan, T., Raja, U. et al. Is procalcitonin a reliable indicator of sepsis in spinal cord injury patients: an observational cohort study. Eur Spine J 32, 1591–1597 (2023). https://doi.org/10.1007/s00586-023-07609-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-023-07609-4