Abstract
Purpose
Recently, competing risk nomograms were widely applied to predict prognosis in numerous tumors other than chordoma. Here, we aimed to construct and validate a competing-risk-based prognostic nomogram to predict 3- and 5-year cancer-specific death (CSD) in patients with spinal and pelvic chordoma.
Methods
All chordoma patient data were abstracted from the Surveillance, Epidemiology, and End Results (SEER) resource, and a total of 485 chordoma patients were eventually included in this study. Multivariate competing risk model and multivariate Cox model were used to determine independent prognostic factors, respectively, and the results of the two models were compared. Nomogram was employed to visualize the competing risk model. The discrimination, calibration, and clinical utility of this model were evaluated by Harrell concordance index (C-index), time-dependent receiver operating characteristic (ROC) curves, calibration plots, and decision curve analysis (DCA). Ten-fold cross-validation was further utilized to validate the prognostic nomogram.
Results
Significant prognostic factors affecting CSD were age (P = 0.016), localized involvement (P < 0.0001), and radical resection (P < 0.001) in the multivariate competing risk model. C-indexes were 0.799 and 0.76, and AUC were 0.812 and 0.778 for 3- and 5-year CSD. Calibration plots demonstrated the nomogram was well-fitted, and DCA indicated good clinical utility. The nomogram showed good performance in the 10-fold cross-validation.
Conclusion
We successfully built the first competing-risk-based nomogram to predict clinical outcomes in patients with spinal and pelvic chordoma. This well-established nomogram hopes to help clinicians with precise prognostic assessment and thus improve clinical outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chordoma arising from notochordal remnants is a relatively rare malignant neoplasm that accounts for 3–4% of all primary bone tumors [1]. The anatomical distribution of chordoma consists mainly of the sacrococcygeal area (55%), the spine region (10%), and the skull area (35%) [2]. Although chordoma is a malignant tumor of low-to-moderate grade, it is noteworthy that chordoma arising in the sacrococcygeal and spinal regions exhibits a more malignant behavior than that in the skull area [3, 4]. In parallel, chordoma in the spinal and sacrococcygeal regions is often delayed in diagnosis due to its rarity, non-specific symptoms, and slow progression [5, 6]. Clinical observations have found that patients with spinal and pelvic chordoma tend to have worse 5- and 10-year overall survival than those with intracranial chordoma [7, 8]. Currently, the relative resistance of spinal and pelvic chordoma to chemotherapeutic agents and the limited success of radiotherapy allow complete en-bloc surgical resection with negative microscopic margins to remain the mainstay of treatment [7, 8]. Unfortunately, locally invasive characteristics, complicated anatomical relationships, and comorbidity considerations make it difficult to achieve surgical resection with negative margins in more than 50% of cases [2, 9]. Hence, the rates of local recurrence and distant metastasis after initial resection of spinal and pelvic chordoma are pretty high, with over 40% of patients occurring distant metastasis after treatment [8, 10, 11]. Furthermore, in the population over 40 years with the highest incidence of spinal and pelvic chordoma, the 10-year survival rate is only 46% [12, 13]. Therefore, there is an urgent need to strengthen research on the prognosis of spinal and pelvic chordoma to guide individualized treatment and follow-up, thereby improving patient survival.
It has been shown that substantial clinical parameters, such as age, fibrinogen, d-dimer, tumor diameter, as well as surgical types, can each independently predict the prognosis of chordoma [14,15,16]. Nomograms, the prognostic device capable of combing multiple parameters that can reveal the relationship between chordoma and prognosis with greater accuracy and convenience than every single parameter that reflects only limited associations with a particular aspect of chordoma, and thus are now widely used for individualized prediction of tumor patients [17, 18]. In the past, nomograms based on traditional Cox proportional hazard regression models and Kaplan–Meier methods have been employed extensively to predict survival outcomes in chordoma patients [16, 19,20,21]. However, it is a non-negligible drawback that competing risk (in other word, non-cause-specific death), such as non-cancer-specific death (NCSD), is treated as censored data in the traditional Cox and Kaplan–Meier survival analyses [22]. Additionally, the competing risk can preclude the occurrence of outcome of interest, thus introducing confusion and bias inevitably in Cox models [23].
Fine and Gray model, also called as competing risk model, was developed to handle survival data for a variety of outcomes including censored, CSD, and NCSD [24]. Plenty of evidence demonstrated that competing risk models are more accurate and stable in predicting outcomes when competing risks are present, compared with the Kaplan–Meier and Cox models [25, 26]. Thus, based on a broad consensus of the literature, the use of the competing risk model is preferentially recommended when one or more competitive risks are present, especially in the middle-aged and elderly population [22, 25]. It is well known that spinal and pelvic chordoma is most prevalent in people over the age of 40, who often have more chronic underlying diseases and therefore have competing risks that should not be ignored. Up to now, competing-risk-based prognostic nomograms have been constructed in remarkable amounts of cancers, such as endometrial cancer, spinal and pelvic chondrosarcoma, maxillary sinus carcinoma, and primary fallopian tube carcinoma [27,28,29,30]. Nonetheless, there is a lack of research on the use of competing risk model to construct nomogram to assess survival outcomes in patients with spinal and pelvic chordoma. We hypothesized that the competing risk model could better predict the outcome of spinal and pelvic chordoma patients.
In this study, we aimed to construct and validate a nomogram based on competing risk model to predict 3- and 5-year CSD in spinal and pelvic chordoma, given their different treatment and characteristics from those of skull base chordoma.
Materials and methods
Study population
In this retrospective cohort study, patients diagnosed with chordoma were extracted from the SEER database. The SEER database collects clinical cancer data in 18 different cancer registries since 1973, covering approximately 30% of the total population in the United States. For this study, patient consent was waived due to this observational study only using the information abstracted from the public SEER database.
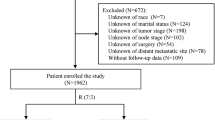
Inclusion criteria were as follows: (1) chordoma diagnosis confirmed by positive histology, rather than biopsy; (2) chordoma diagnosed from 2003 to 2016, with vertebral column and pelvic bone as the primary site; (3) known months of survival. The exclusion criteria were: (1) unknown cause of death, including CSD and NCSD; (2) patients with survival time ≤ 1 month, because they could not be included in the competing risk model; (3) patients with missing or incomplete demographic and tumor information, including age, race, sex, marital, site, chemotherapy, range, subtype, radiotherapy, and surgery; and (4) patients with other malignant tumors. Ultimately, 485 patients were eligible for final analysis. The flowchart of inclusion and exclusion processes is shown in Fig. 1. Demographic and tumor information of patients with spinal and pelvic chordoma were obtained through the SEER*Stat software (version 8.4.0).
Variable selection and outcomes
The demographic variables, such as age at diagnosis, race, sex, and marital status, were included. Tumor characteristics consist of primary site, histologic type, and tumor range. Treatment strategies of the patients, including radiotherapy, chemotherapy, as well as surgery, were required. Age was obtained from the “Age recode with single ages and 100 + ” field and treated as one continuous variable. Race was determined according to the “Race recode (W, B, AI, API)” field and classified into three groups, including white, black, as well as other (American Indian/Alaska Native and Asian or Pacific Islander). Site retrieved from the “Site and Morphology Primary site-labeled” field was divided into the spine (C41.2-Vertebral column) and pelvis (C41.4-Pelvic bones, sacrum, coccyx, and associated joints). According to the International Classification of Disease for Oncology, 3rd Edition (ICD-O-3) code, histologic type was divided into 3 subtypes: not otherwise specified (NOS, code 9370), chondroid (code 9371), as well as dedifferentiated (code 9372). Surgical types were extracted according to the “RX Summ-Surg Prim Site (1998 +)” field and further categorized into three categories: no surgery (code 0), subtotal resection (codes 15, 19, 25, as well as 26), and radical resection (codes 30, 40, 41, 42, 50, 51, 52, 53, as well as 54). Codes 90 and 99 were treated as missing information. In the “SEER historic stage A (1973–2015)” field, primary tumor range was recorded as localized, regional, as well as distant. However, there existed many missing data in this field (n = 100, 17.6%). The “SEER Combined Summary Stage 2000 (2004–2017)” field and M stage in the “Derived AJCC M, 6th ed (2004–2015)” field were consulted to impute missing data in this field. Finally, missing values still existed in some variables, including race (n = 8, 1.4%), range (n = 31, 5.5%), radiotherapy (n = 16, 2.8%), and surgery (n = 17, 3.0%). Patients with missing data were excluded.
Survival information assessed included CSD, NCSD, and survival months. CSD regarded as death due to chordoma was the primary outcome of interest in the present study. Deaths resulting from causes other than chordoma were determined as NCSD. CSD was extracted according to the “SEER cause-specific death classification” field and recorded as “Alive or dead of other cause” and “Dead (attributable to this cancer dx)” in the SEER database. NCSD was extracted according to the “SEER other cause of death classification” field and recorded as “Alive or dead due to cancer” and “Dead (attributable to causes other than this cancer dx)” in the SEER database. Survival time was deemed as the survival span from diagnosis of spinal and pelvic chordoma to death of any cause or censored.
Construction and validation of the nomogram
A total population of 485 patients was employed to build the competing risk model for CSD. Cumulative incidence function (CIF) curve was calculated to assess the incidence of CSD and competing risk for 3 and 5 years. CIF subgroup analyses were further performed, and the Fine and Gray test was adopted to explore the differences between the subgroup of all kinds of variables. Multivariate competing risk model was carried out to determine the possible prognostic variables. Multivariate Cox regression analysis was also performed for comparison. SHR was utilized to estimate the associations of patient characteristics with CSD. Factors that were of clinical significance in the multivariate analysis were adopted to build the nomogram.
The predictive performance of the prognostic nomogram was further evaluated. C-index, ROC curves, area under the curve (AUC), as well as calibration plots, were employed to evaluate the discrimination and calibration of the prognostic nomogram, while DCA was employed to measure the clinical utility. For the C-index, value 1 showed perfect discriminative performance, and value 0.5 showed random chance [31]. Ten-fold cross-validation, widely utilized to validate a model, was employed to assess the generalizability of the nomogram [32].
Statistical analyses
R-software (version 4.1.2) was employed for all statistical tests. “cmprsk”, “survival”, and “riskRegression” packages were used in the univariate and multivariate analysis. Packages, including “mstate”, and “regplot”, were used for nomogram construction. Packages, including “prodlim”, “pec”, and “QHScrnomo”, were used for prognostic nomogram validation. The step-by-step details about competing risk model construction and validation could refer to Lin et al. [33]. Continuous variable (age) was reported as mean + standard deviation (SD), and categorical data, such as race, sex, as well as subtype, were expressed as frequencies and percentages. Race and subtype were evaluated by Fisher's exact test, and other categorical variables were analyzed by chi-squared test. Multivariate competing risk model was used to identify the independent prognostic factors. In subgroup analyses, the differences were tested by Gray’s test. The final prognostic nomogram included predictors that were of clinical significance in the multivariate analysis. P value of two-sided < 0.05 was regarded as statistical significance.
Results
Patient characteristics
The detailed characteristics of patients were listed in Table 1. Among the 485 cases, 113 (23.3%) patients died of chordoma, and 99 (20.4%) patients died of causes other than chordoma. NCSD accounts for 46.7% of total death, and CSD accounts for 53.3% of total death, indicating that NCSD had great impact on the survival of patients with spinal or pelvic chordoma. The median follow-up period was 66 (39–102) months. The 3-year as well as 5-year cumulative incidence of CSD were 11.0% (95% CI: 8.4–14.0%) and 15.8% (95% CI: 12.6–19.3%), by the competing risk model. Variables, including age, range, as well as surgical types, differed significantly between the censored, CSD as well as NCSD patients (P < 0.001 for all). The mean ages at diagnosis were 54.8 ± 17.0 years, 64.9 ± 17.1 years, and 71.9 ± 13.9 years in the censored, CSD, and NCSD groups. The majority of patients were white (87.8%). NOS was the most prevalent cancer subtype (96.3%), followed by chondroid (2.3%), and dedifferentiated (1.4%). The distribution of radiotherapy was no (48.7%) and yes (51.3%), respectively, while only 5.2% of the total study population received chemotherapy, probably attributed to the relatively limited efficacy of traditional chemotherapeutic agents. Of the 485 chordoma cases, 115 (23.7%) were treated with no surgery, 191 (39.4%) were treated with radical resection, and 179 (36.9%) were treated with subtotal resection.
CIF survival analysis
The CIF curves and P values analyzed by subgroup analysis are shown in Fig. 2. The results showed that patients with age older than 50 years (Fig. 2A), dedifferentiated subtype (Fig. 2E), distant involvement (Fig. 2F), received chemotherapy and did not undergo surgery were positively associated with the incidence of CSD. However, patients with age lower than 50 years (Fig. 2A) as well as those who did not receive surgery (Fig. 2I) were significantly linked to increased incidence of NCSD (P < 0.001 for both). Additionally, female, alone, and pelvis chordoma patients were correlated with a higher cumulative incidence level of CSD, with no significant difference (P > 0.05). However, radiotherapy was not statistically significantly correlated with CSD and NCSD (Fig. 2G).
The CIF curves of CSD and NCSD in patients with spinal and pelvic chordoma. A age; B sex; C marital; D site; E subtype; F range; G radiotherapy; H chemotherapy; I surgical types. In each panel, the solid line indicated CSD, while the dotted line designated NCSD. For every factor, the P value of the Fine and Gray test was calculated. P = 0 in Figures E, F and I represented P value < 0.0001
Multivariate analyses by competing risk model and Cox model
To further determine the prognostic factors of patients with spinal and pelvic chordoma, we performed multivariate competing risk analyses in the total population. As shown in Table 2, significant prognostic factors affecting CSD in the proportional sub-distribution hazards regression model were age (sub-distribution hazards ratio [SHR]: 1.015, 95% confidence interval [CI]: 1.003–1.028, P = 0.016), localized involvement (SHR: 0.275, 95% CI: 0.151–0.504, P < 0.0001), and radical resection (SHR: 0.385, 95% CI: 0.221–0.670, P < 0.001). No significant differences were identified between the pelvis and spine (P = 0.050). Compared with patients who did not treat with radiotherapy, the SHR of those who received radiotherapy was 0.950 (95% CI 0.634–1.424), with no significant difference (P = 0.80). Subsequently, multivariate Cox proportional hazards model was also performed for comparison. The Cox regression analysis determined that older age (hazard ratio [HR]: 1.026, 95% CI: 1.013–1.040, P < 0.001), site (HR: 1.711, 95% CI:1.127–2.598, P = 0.012), localized involvement (HR: 0.226, 95% CI: 0.126–0.406, P < 0.0001), as well as radical resection (HR: 0.272, 95% CI: 0.154–0.480, P < 0.0001) were independent predictors.
Nomogram construction and validation
The significant variables identified in the multivariate competing risk model were included in the nomogram. Finally, a nomogram constructed by three meaningful variables, including age, range, and surgical types, was used to visualize the 3-year and 5-year probability of CSD for patients with spinal and pelvic chordoma (Fig. 3).
Nomogram based on competing risk model for predicting the 3-year and 5-year probabilities of CSD in spinal or pelvic chordoma. Take for an example. One 55-year-old patient with regional involvement was treated with radical resection. He gained cumulative point scores of 98.6 by adding up all the scores obtained in age, range, and surgical types. Correspondingly, the probabilities of CSD of 3- and 5-year were 0.0369 and 0.066
The C-indexes of the nomogram for evaluating the 3- and 5-year probability of CSD were 0.799 and 0.76. AUC of this competing risk-based nomogram for predicting 3- and 5-year likelihoods of CSD were 0.812 (95% CI: 0.748–0.876, Fig. 4A) and 0.778 (95% CI: 0.711–0.846, Fig. 4B), respectively, indicating excellent discrimination ability of this present model. As shown in the calibration graphs, curves for 3-year (Fig. 4C) and 5-year (Fig. 4D) CSD exhibited relatively perfect agreement between observed and predicted probabilities. The high clinical application value of the prognostic nomogram was confirmed by DCA (Fig. 4E and F). When the threshold probability was between about 0.05 and 0.45, net benefit could be obtained. Finally, to assess the generalizability of the model, 10-fold cross-validation was conducted. The C-index was 0.688 in the internal validation, indicating relatively strong stability of the prognostic nomogram.
Time-dependent ROC curves, calibration plots, and DCA for the competing risk model-based nomogram. A ROC curves of the nomogram in predicting 3-year CSD; B ROC curves of the nomogram in predicting 5-year CSD; C calibration plots of the nomogram in predicting 3-year CSD; D calibration plots of the nomogram in predicting 5-year CSD; E DCA of the nomogram in predicting 3-year CSD; F DCA of the nomogram in predicting 5-year CSD
Discussion
Herein, we successfully constructed and validated a competing risk model-based nomogram built by three predictors for predicting the 3- and 5-year CSD in spinal and pelvic chordoma patients. The final model showed relatively well discrimination, calibration, and clinical utility in the internal validation, with the C-indexes of 0.799 and 0.76 for 3- and 5-year CSD. Take an example of using the prognostic nomogram. One 55-year-old patient with regional involvement was treated with radical resection. He gained cumulative point scores of 98.6 by adding up all the scores obtained in age, range, and surgical types. Correspondingly, the probabilities of CSD of 3- and 5-year were 0.0369 and 0.066.
Independent predictors of CSD, including age at diagnosis, sex, race, stage, surgical types, year of diagnosis, marital status, primary site, histological type, radiation therapy, and tumor size, were identified for spinal and pelvic chordoma patients based on Cox and Kaplan–Meier models in previous studies [16, 19, 20]. However, after selection by competing risk model, factors that influenced the probability of CSD were age, range, and surgical types in the final model, unlike the results determined by traditional Cox and Kaplan–Meier analyses. Different results may be attributed to differences between the two models. In traditional Cox and Kaplan–Meier models, competing risk is considered censored and the impact of competing risk on events of interest is ignored, thus may inaccurately predict the probability of interest events. Notably, numerous meta-analyses comparing Cox and Kaplan–Meier models with competing risk models confirm that the Cox and Kaplan–Meier methods overestimated the cumulative incidence of interest event in the presence of competing risk and that the use of competing risk models in such cases ensured more accurate results [26, 34]. To demonstrate the differences between the two models in predicting the prognosis of patients with chordoma, we also provide the nomogram constructed by traditional Cox and Kaplan–Meier methods. As shown in supplementary Fig. 1, one 55-year-old patient with regional involvement who was treated with radical resection, the probabilities of CSD of 3- and 5-year were 0.0446 and 0.0717, respectively. Compared with the result obtained from the competing risk nomogram (Fig. 3), the probabilities of CSD of 3- and 5-year were 0.0369 and 0.066. This further confirmed that the Cox and Kaplan–Meier model overestimated the cumulative incidence of CSD in the presence of competing risk in chordoma patients.
Besides, compared with the previous Cox model, although only three factors are included in the competing risk model, it has comparable C-indexes that 0.799 for 3-year CSD and 0.76 for 5-year CSD. Huang et al. [16] reported the C-index of a nomogram based on Cox and Kaplan–Meier models for predicting 3-, 5-, and 10-year survival in spinal chordoma patients was 0.76. Similarly, Meng et al. [20] showed the C-indexes of the Cox model-based nomogram for predicting local relapse-free survival and overall survival were 0.79 and 0.76. Therefore, this competing-risk-based nomogram is recommended when one plans to predict the 3- and 5-year probabilities of CSD in patients with spinal and pelvic chordoma.
Consistent with previous studies [16], our study showed that patients who were older than 50 years, did not undergo surgery, and had distant involvement were more likely to have worse outcomes. Of the three independent predictors, age (P < 0.001) and surgical types (P < 0.001) were significantly associated with both CSD and NCSD in the CIF analysis. As we know, older patients are more likely to die from NCSD, such as heart disease and chronic obstructive pulmonary disease, which is competing risk in the competing risk model [35]. In our study, NCSD accounts for a relatively large proportion of total death (46.7%), nearly equal to CSD (53.3%), suggesting that NCSD has a great role in the prognosis of chordoma patients. Therefore, NCSD should be taken into account when one planned to predict the outcome of spinal and pelvic chordoma patients. This further highlighted the equal importance of meticulous management to avoid NCSD, particularly in elderly chordoma patients. The extent of surgical resection was another key predictor in determining clinical outcomes in the present model. Advances in surgical techniques in recent years, such as arterial embolization technology, are beneficial to facilitate surgical resection [36]. Nonetheless, complete en-bloc surgical resection with negative margins is still a major challenge. We can see that in the current research. Among the 485 recipients, only 191 cases (39.4%) were treated with radical resection. Consistent with our study, several studies indicated that surgery with negative margins was still the preferred treatment to achieve local control and a well long-term outcome [37]. Besides, a retrospective cohort study by Zuckerman et al. [38] certified that although complete en-bloc surgical resection with negative margins was not statistically significantly correlated with overall survival, it was significantly associated with lower levels of local recurrence in patients with sacral chordoma. More studies are still needed in the future to verify the prognostic importance of surgical types for spinal and pelvic chordoma. In addition, previous studies have shown that chordoma is inherently resistant to conventional chemotherapy and radiotherapy [39]. This finding is consistent with our results that neither chemotherapy nor radiotherapy were independent prognostic factors in multivariate Cox or competing risk models. Surprisingly, in our series, individuals receiving chemotherapy showed a higher cumulative incidence level of CSD, possibly the indication of an advanced stage of the tumor, making them unfit for complete surgical resection.
This competing-risk-based nomogram has the following strengths. First of all, it is noteworthy that for the first time, we constructed a competing risk model-based nomogram for patients with spinal and pelvic chordoma. In addition, factors (age, range, as well as surgical types) included in the final model were conventional clinical parameters and readily available. Besides, our model built by a relatively small number of prognostic factors has a quite good performance in predicting 3- and 5-year CSD, compared with several models previously constructed by other investigators. Moreover, chordoma is a rare tumor, and the cases of previous studies are always limited to a single center, so it is difficult to construct a prognostic nomogram in a limited population and even introduces confusion and bias, making the study results inaccurate [40]. By contrast, the SEER database, which covers a large number of cancer patients [41], was used in this study, and 485 cases were included in the final analysis, which is a relatively large cohort compared to numerous previous studies. Additionally, unlike other studies that simply delete the censored information, a large portion of missing values in this study was imputed by consulting the related field in the database. Furthermore, another strength of the present study is that DCA was also employed to estimate the net benefit of the nomogram. Collectively, using this nomogram with relatively high predictive performance is expected to reduce the burden of spinal and pelvic chordoma by risk stratification and personalized treatments [42].
This study also has certain limitations. Firstly, tumor size has been identified as an independent prognostic factor in endometrial cancer and somewhat suggests a possible correlation with survival in patients with chordoma [27]. Due to the large percentage of missing information (39.2% in the original data), the present study, unfortunately, did not include the above indicator. Secondly, chemotherapy retrieved from the database was defined as “yes” and “no/unknown”, but we did not know the exact information about “no/unknown”, thus probably introducing significant bias. Thirdly, all the data were extracted from the SEER database, external validation did not carry out, but with ten-fold cross-validation in the internal validation. Finally, the present research was designed as a retrospective study with inherent selection bias.
Conclusion
We successfully constructed and internally validated a competing risk model-based prognostic nomogram for predicting the 3- and 5-year CSD in spinal and pelvic chordoma patients using public data abstracted from the SEER database. This simple but accurate tool can help clinicians with precise prognostic assessment and thus improve survival outcomes.
Data availability
The data that support the findings of this study are freely available on SEER database.
Abbreviations
- CSD:
-
Cancer-specific death
- SEER:
-
Surveillance, epidemiology, and end results
- C-index:
-
Harrell concordance index
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under the curve
- DCA:
-
Decision curve analysis
- SHR:
-
Sub-distribution hazards ratio
- CI:
-
Confidence interval
- NCSD:
-
Non-cancer-specific death
- ICD-O-3:
-
International classification of disease for oncology, 3rd Edition
- NOS:
-
Not otherwise specified
- AJCC:
-
American joint committee on cancer
- CIF:
-
Cumulative incidence function
- HR:
-
Hazard ratio
- SD:
-
Standard deviation
References
Yeh CY (2021) Radiotherapy of extraosseous nasopharyngeal chordoma: a case report and literature review. Mol Clin Oncol 14:84. https://doi.org/10.3892/mco.2021.2246
Zuckerman SL, Bilsky MH, Laufer I (2018) Chordomas of the skull base, mobile spine, and sacrum: an epidemiologic investigation of presentation, treatment, and survival. World Neurosurg 113:e618–e627. https://doi.org/10.1016/j.wneu.2018.02.109
Murphey MD, Andrews CL, Flemming DJ, Temple HT, Smith WS, Smirniotopoulos JG (1996) From the archives of the AFIP. Primary tumors of the spine: radiologic pathologic correlation. Radiographics: a review publication of the Radiological Society of North America Inc 16:1131–1158. https://doi.org/10.1148/radiographics.16.5.8888395
Bjornsson J, Wold LE, Ebersold MJ, Laws ER (1993) Chordoma of the mobile spine. A clinicopathologic analysis of 40 patients. Cancer 71:735–740. https://doi.org/10.1002/1097-0142(19930201)71:3%3c735::aid-cncr2820710314%3e3.0.co;2-8
Wedekind MF, Widemann BC, Cote G (2021) Chordoma: current status, problems, and future directions. Curr Prob Cancer 45:100771. https://doi.org/10.1016/j.currproblcancer.2021.100771
Gokaslan ZL, Zadnik PL, Sciubba DM, Germscheid N, Goodwin CR, Wolinsky JP, Bettegowda C, Groves ML, Luzzati A, Rhines LD, Fisher CG, Varga PP, Dekutoski MB, Clarke MJ, Fehlings MG, Quraishi NA, Chou D, Reynolds JJ, Williams RP, Kawahara N, Boriani S (2016) Mobile spine chordoma: results of 166 patients from the AOSpine knowledge forum tumor database. J Neurosurg Spine 24:644–651. https://doi.org/10.3171/2015.7.Spine15201
Pennington Z, Ehresman J, McCarthy EF, Ahmed AK, Pittman PD, Lubelski D, Goodwin CR, Sciubba DM (2021) Chordoma of the sacrum and mobile spine: a narrative review. Spine J 21:500–517. https://doi.org/10.1016/j.spinee.2020.10.009
Stacchiotti S, Sommer J (2015) Building a global consensus approach to chordoma: a position paper from the medical and patient community. Lancet Oncol 16:e71-83. https://doi.org/10.1016/s1470-2045(14)71190-8
Clarke MJ, Dasenbrock H, Bydon A, Sciubba DM, McGirt MJ, Hsieh PC, Yassari R, Gokaslan ZL, Wolinsky JP (2012) Posterior-only approach for en bloc sacrectomy: clinical outcomes in 36 consecutive patients. Neurosurgery 71:357–364; discussion 364. https://doi.org/10.1227/NEU.0b013e31825d01d4
George B, Bresson D, Herman P, Froelich S (2015) Chordomas: a review. Neurosurg Clin N Am 26:437–452. https://doi.org/10.1016/j.nec.2015.03.012
Moojen WA, Vleggeert-Lankamp CL, Krol AD, Dijkstra SP (2011) Long-term results: adjuvant radiotherapy in en bloc resection of sacrococcygeal chordoma is advisable. Spine (Phila Pa 1976) 36:E656-661. https://doi.org/10.1097/BRS.0b013e3181f8d1f3
Jabbar R, Jankowski J, Pawelczyk A, Szmyd B, Solek J, Pierzak O, Wojdyn M, Radek M (2022) Cervical paraspinal chordoma: a literature review with a novel case report. J Clin Med. https://doi.org/10.3390/jcm11144117
Kurup AN, Woodrum DA, Morris JM, Atwell TD, Schmit GD, Welch TJ, Yaszemski MJ, Callstrom MR (2012) Cryoablation of recurrent sacrococcygeal tumors. J Vasc Interv Radiol 23:1070–1075. https://doi.org/10.1016/j.jvir.2012.05.043
Li M, Bai J, Wang S, Zhai Y, Zhang S, Li C, Du J, Zhang Y (2020) Prognostic value of cumulative score based on preoperative fibrinogen and albumin level in skull base chordoma. Onco Targets Ther 13:8337–8346. https://doi.org/10.2147/ott.S257779
Li B, Zhang H, Zhou P, Yang J, Wei H, Yang X, Yang C, Wu Z, Xiao J (2019) Prognostic significance of pretreatment plasma D-dimer levels in patients with spinal chordoma: a retrospective cohort study. Eur Spine J 28:1480–1490. https://doi.org/10.1007/s00586-018-05872-4
Huang JF, Chen D, Sang CM, Zheng XQ, Lin JL, Lin Y, Ni WF, Wang XY, Li YM, Wu AM (2019) Nomogram for individualized prediction and prognostic factors for survival in patients with primary spinal chordoma: a population-based longitudinal cohort study. World Neurosurg 128:e603–e614. https://doi.org/10.1016/j.wneu.2019.04.217
Balachandran VP, Gonen M, Smith JJ, DeMatteo RP (2015) Nomograms in oncology: more than meets the eye. Lancet Oncol 16:e173-180. https://doi.org/10.1016/s1470-2045(14)71116-7
Chen D, Liu Z, Liu W, Fu M, Jiang W, Xu S, Wang G, Chen F, Lu J, Chen H, Dong X, Li G, Chen G, Zhuo S, Yan J (2021) Predicting postoperative peritoneal metastasis in gastric cancer with serosal invasion using a collagen nomogram. Nat Commun 12:179. https://doi.org/10.1038/s41467-020-20429-0
Huang Z, Fan Z, Zhao C, Sun H (2021) A novel nomogram for predicting cancer-specific survival in patients with spinal chordoma: a population-based analysis. Technol Cancer Res Treat 20:15330338211036532. https://doi.org/10.1177/15330338211036533
Meng T, Huang R, Hu P, Yin H, Lin S, Qiao S, Wang R, Wang J, Cai Z, Huang Z, Cheng L, Song D (2021) Novel nomograms as aids for predicting recurrence and survival in chordoma patients: a retrospective multicenter study in mainland China. Spine (Phila Pa 1976) 46:E37-e47. https://doi.org/10.1097/brs.0000000000003716
Teng C, Yang Q, Xiong Z, Ye N, Li X (2021) Multivariate analysis and validation of the prognostic factors for skull base chordoma. Front Surg 8:764329. https://doi.org/10.3389/fsurg.2021.764329
Austin PC, Lee DS, Fine JP (2016) Introduction to the analysis of survival data in the presence of competing risks. Circulation 133:601–609. https://doi.org/10.1161/circulationaha.115.017719
Nolan EK, Chen HY (2020) A comparison of the Cox model to the fine-Gray model for survival analyses of re-fracture rates. Arch Osteoporos 15:86. https://doi.org/10.1007/s11657-020-00748-x
Fine JP, Gray RJ (1999) A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94:496–509. https://doi.org/10.2307/2670170
de Glas NA, Kiderlen M, Vandenbroucke JP, de Craen AJ, Portielje JE, van de Velde CJ, Liefers GJ, Bastiaannet E, Le Cessie S (2016) Performing survival analyses in the presence of competing risks: a clinical example in older breast cancer patients. J Nat Cancer Instit. https://doi.org/10.1093/jnci/djv366
Lacny S, Wilson T, Clement F, Roberts DJ, Faris P, Ghali WA, Marshall DA (2018) Kaplan-Meier survival analysis overestimates cumulative incidence of health-related events in competing risk settings: a meta-analysis. J Clin Epidemiol 93:25–35. https://doi.org/10.1016/j.jclinepi.2017.10.006
Xie G, Qi C, Yang W, Wang R, Yang L, Shang L, Huang L, Chung MC (2021) Competing risk nomogram predicting cancer-specific mortality for endometrial cancer patients treated with hysterectomy. Cancer Med 10:3205–3213. https://doi.org/10.1002/cam4.3887
Dong Y, Xie L, Kang H, Peng R, Guo Q, Song K, Wang J, Guan H, Fang Z, Li F (2021) A competing risk-based prognostic model to predict cancer-specific death of patients with spinal and pelvic chondrosarcoma. Spine (Phila Pa 1976) 46:E1192-e1201. https://doi.org/10.1097/brs.0000000000004073
Hu M, Li X, Gu W, Mei J, Liu D, Chen S (2021) A competing risk nomogram for predicting cancer-specific death of patients with maxillary sinus carcinoma. Front Oncol 11:698955. https://doi.org/10.3389/fonc.2021.698955
Li C, Li J, Huang Q, Feng X, Zhao F, Xu F, Han D, Lyu J (2021) Developing and validating a novel nomogram used a competing-risks model for predicting the prognosis of primary fallopian tube carcinoma: a retrospective study based on the SEER database. Ann Transl Med 9:378. https://doi.org/10.21037/atm-20-5398
Harrell FE Jr, Lee KL, Mark DB (1996) Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15:361–387. https://doi.org/10.1002/(sici)1097-0258(19960229)15:4%3c361::Aid-sim168%3e3.0.Co;2-4
Gittleman H, Sloan AE, Barnholtz-Sloan JS (2020) An independently validated survival nomogram for lower-grade glioma. Neuro Oncol 22:665–674. https://doi.org/10.1093/neuonc/noz191
Lin H, Zheng H, Ge C, Ling L, Yin R, Wang Q, Zhang X, Zhou S, Jin X, Xu X, Fu J (2022) An R-based landscape validation of a competing risk model. J Vis Exp : JoVE. https://doi.org/10.3791/64018
Lacny S, Wilson T, Clement F, Roberts DJ, Faris PD, Ghali WA, Marshall DA (2015) Kaplan-Meier survival analysis overestimates the risk of revision arthroplasty: a meta-analysis. Clin Orthop Relat Res 473:3431–3442. https://doi.org/10.1007/s11999-015-4235-8
Wu L, Ge C, Zheng H, Lin H, Fu W, Fu J (2020) Establishing a competing risk regression nomogram model for survival data. J Vis Exp: JoVE. https://doi.org/10.3791/60684
Griessenauer CJ, Salem M, Hendrix P, Foreman PM, Ogilvy CS, Thomas AJ (2016) Preoperative embolization of spinal tumors: a systematic review and meta-analysis. World Neurosurg 87:362–371. https://doi.org/10.1016/j.wneu.2015.11.064
Colangeli S, Muratori F, Bettini L, Frenos F, Totti F, D’Arienzo A, Campo FR, Scoccianti G, Beltrami G, Campanacci DA, Capanna R (2018) Surgical treatment of sacral chordoma: en bloc resection with negative margins is a determinant of the long-term outcome. Surg Technol Int 33:343–348
Zuckerman SL, Lee SH, Chang GJ, Walsh GL, Mehran RJ, Gokaslan ZL, Rao G, Tatsui CE, Rhines LD (2021) Outcomes of surgery for sacral chordoma and impact of complications: a report of 50 consecutive patients with long-term follow-up. Global Spine J 11:740–750. https://doi.org/10.1177/21925682211011444
Ulici V, Hart J (2022) Chordoma. Arch Pathol Lab Med 146:386–395. https://doi.org/10.5858/arpa.2020-0258-RA
Hobusch GM, Bodner F, Walzer S, Marculescu R, Funovics PT, Sulzbacher I, Windhager R, Panotopoulos J (2016) C-reactive protein as a prognostic factor in patients with chordoma of lumbar spine and sacrum–a single center pilot study. World J Surg Oncol 14:111. https://doi.org/10.1186/s12957-016-0875-8
Wang J, Yang Y, Pan J, Qiu Y, Shen S, Wang W (2022) Competing-risk nomogram for predicting survival in patients with advanced (stage III/IV) gallbladder cancer: a SEER population-based study. Jpn J Clin Oncol 52:353–361. https://doi.org/10.1093/jjco/hyab212
Bibault JE, Chang DT, Xing L (2021) Development and validation of a model to predict survival in colorectal cancer using a gradient-boosted machine. Gut 70:884–889. https://doi.org/10.1136/gutjnl-2020-321799
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
HL designed the current study and amended the paper. JL and CM were the primary writer of the paper and were responsible for the statistical analysis. JL made critical revisions to the manuscript and created all tables and figures. CM revised the English language. JL, CM, XY, NL, YX, and JG performed the literature search and data collection. All authors approved the final version of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no conflict of interests.
Ethics approval
Ethics approval was waived due to this observational study only using the information abstracted from the public SEER database.
Consent for publication
All the authors listed have approved the manuscript for publication in European Spine Journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, J., Ma, C., Yuan, X. et al. Competing risk nomogram for predicting prognosis of patients with spinal and pelvic chordoma: A SEER-based retrospective study. Eur Spine J 32, 1334–1344 (2023). https://doi.org/10.1007/s00586-023-07590-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-023-07590-y