Abstract
Objective
Dysphagia is the most commonly reported complication of annterior cervical discectomy and fusion (ACDF) surgery. However, the incidence of dysphagia post-ACDF varies widely–partly attributable to differing outcome measures used to capture dysphagia. Our objective was to conduct a scoping review of the literature to quantify which dysphagia outcome measures have been employed post-ACDF and examine trends by study design, year, and location.
Methods
After removing duplicates, 2396 abstracts were screened for inclusion. A total of 480 studies were eligible for full-text review. After applying exclusion criteria, data was extracted from 280 studies. We extracted the dysphagia outcome measure(s), study design (prospective vs retrospective), year, and location (country). Approximately 10% of studies were repeated for intra-rater agreement.
Results
In total, 317 dysphagia outcome measures were reported in 280 studies (primarily retrospective—63%). The largest proportion of outcome measures were categorized as “unvalidated patient-reported outcome measures” (46%), largely driven by use of the popular Bazaz scale. The next most common categories were “insufficient detail” and “validated patient-reported outcome measures” (both 16%) followed by “chart review/database” (13%) and instrumental assessment (7%). Studies examining dysphagia post-ACDF steadily increased over the years and the use of validated measures increased in the past 10 years.
Conclusions
This scoping review of the literature highlights that nearly half of the ACDF dysphagia literature relies on unvalidated patient-reported outcome measures. The current understanding of the mechanism, timeline, and presentation of dysphagia post-ACDF are likely limited due to the metrics that are most commonly reported in the literature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Swallowing is a complex, highly coordinated sensory-motor sequence that most people take for granted. Yet, in order to execute a safe and efficient swallow, 25 pairs of muscles controlled by five cranial nerves contract and relax in rapid succession, all intricately timed within the respiratory cycle [1]. Dysphagia (disordered swallowing) can result in food/liquid being misdirected into the respiratory tract (a process known as aspiration) and can cause pneumonia [2]. Weak and/or or poorly coordinated swallowing can result in inefficient swallowing, further contributing to aspiration risk and cause challenges with meeting nutrition and hydration needs [3, 4]. In extreme cases, patients no longer eat and drink by mouth and receive nutrition and hydration via enteral feeding. In addition to the impact on the patient’s health, dysphagia is known to significantly impact quality of life [5], healthcare costs [6, 7], length of hospital stay [8], and caregiver burden [9]. In North America, speech language pathologists (SLPs) and otolaryngologists are the healthcare professionals typically responsible for assessing swallowing.
Dysphagia after ACDF
Dysphagia is the most commonly reported complication of anterior cervical discectomy and fusion (ACDF) surgery [10]. Dysphagia after ACDF surgery can result from postsurgical edema [11] and/or neuropraxia from nerve compression or retraction [12]. The intubation injuries are also known to increase risk of dysphagia [13]. Depending on which cervical levels are involved, the ACDF surgery places several important swallowing nerves and structures at iatrogenic risk. For example, the hypoglossal nerve is at risk at C2–C3 [14] and innervates nearly all the extrinsic and intrinsic muscles of the tongue. These muscles are critical for the formation and propulsion of the bolus. The internal branch of the superior laryngeal nerve (iSLN) is at risk near C3–4 and is responsible for sensory innervation of the laryngeal mucosa above the vocal folds [14]. The impairment of the iSLN can place an individual at risk of aspiration, given that food and liquid is not sensed entering the laryngeal vestibule. The recurrent laryngeal nerve (RLN) innervates nearly all the intrinsic laryngeal muscles and is instrumental in positioning the vocal folds for phonation and airway protection. The RLN is at risk of injury at the level of C6–C7 and also from surgical retraction [15]. Finally, many muscles that are important to swallowing are also at risk during ACDF surgery such as the pharyngeal constrictors (responsible for bolus propulsion) and the posterior digastric muscle (integral for airway protection during swallowing).
The reported incidence of dysphagia after ACDF is notoriously wide, with recent systematic reviews suggesting a range of 1–79% [16] and 0.2–87.5% [17]. While many reasons have been investigated to explain the variance in dysphagia incidence (such as time since surgery, age, sex, co-morbidities, cervical surgical level(s), surgical approach, operating room time, hardware type, use of steroids, etc.), it cannot be ignored that the choice of dysphagia metric contributes to this wide range [18,19,20]. Ideally, dysphagia risk is either identified with screening tools and patient-reported outcome measures that are psychometrically valid and/or dysphagia is confirmed using gold-standard instrumental assessment of swallowing. Two widely adopted techniques for visualizing swallowing are flexible endoscopic evaluation of swallowing (FEES) and videofluoroscopic swallowing studies (VFSS), also known as modified barium swallow studies (MBS). Most swallowing specialists consider the latter to be the gold-standard assessment [21]. Yet, the ACDF literature appears to rely heavily on non-validated patient-reported outcomes to capture dysphagia—which may challenge what is confidently known about dysphagia in this population. It is currently unknown how pervasive this issue is. Therefore, our primary goal was to conduct a scoping review of the literature to quantify which dysphagia outcome measures have been reported in the ACDF literature. Our secondary goals were to explore trends over time, by study design, and study location.
Methods
Following guidance outlined by Munn and colleagues, we determined that our research goals were aligned with a scoping review (as opposed to a systematic review) [22]. A scoping review sets out to identify patterns of evidence using an a priori review protocol; however, unlike a systematic review, it does not include a mandatory risk of bias assessment. Our goals are consistent with three of six possible purposes Munn and colleagues identified for conducting a scoping review including “to identify the types of available evidence in a given field, to clarify definitions in the literature, to identify and analyze knowledge gaps” [22]. In March 2022, we searched three medical databases (ProQuest Nursing & Allied Health, PUBMED, Scopus) using the search terms (“anterior cervical discectomy fusion”) OR (“ACDF”) OR (“cervical spine surgery”) AND (“dysphagia”). Our goal was to locate all empirical studies published in English that reported dysphagia outcomes after planned, initial ACDF surgery. Systematic reviews, case studies, revision surgeries, non-ACDF surgeries, and trauma populations were not included. The quality of individual studies was not appraised for this review.
First, titles and abstracts were screened for exclusion. Next, the full-text versions were reviewed to confirm inclusion. Finally, data were extracted from the final set of articles. Parameters of interest included dysphagia outcome measure(s), study year, study design (retrospective vs prospective), and study location (country per final author’s affiliation). When dysphagia was quantitatively reported as a complication of ACDF surgery in a given publication but a metric was not described in the methods section, studies were coded as “insufficient detail.” Data analysis was conducted by trained speech language pathology (SLP) students. Approximately 10% of studies were reviewed by a second rater (SLP with 5 years clinical experience) at each step (title and abstract review, full-text review) to calculate percent exact agreement.
Results
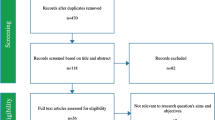
The process of data extraction and refinement is summarized in Fig. 1. Results from our database searches were imported into Covidence (Veritas Health Innovation) (n = 4716). After duplicates were removed, 2396 abstracts and titles were screened for inclusion. Percent agreement (on 200 abstract/titles) was high at this step (92.5%). Discrepancies were resolved by the first author. After screening titles and abstracts, 480 full-text studies were reviewed to confirm inclusion. A total of 200 studies were excluded for the following reasons: no dysphagia outcomes (n = 81), study design (n = 53), study not published in English (n = 36), wrong intervention (n = 24) and trauma population (n = 6). As a result, we were able to extract parameters of interest from 280 studies. Our primary variable of interest was the number and type of dysphagia outcome measures reported, followed by secondary variables of interest including study year, study design (prospective vs retrospective), and study country. Data extraction was repeated by an experienced SLP rater in 20 studies and yielded 90% agreement. Again, discrepancies were resolved by the first author.
Outcome measures
Our review revealed that there were 317 dysphagia outcome measures reported in these 280 studies with the majority of studies reporting one outcome measure (n = 251), with fewer studies reporting two (n = 22), three (n = 6), or four (n = 1) dysphagia outcome measures. The full list of possible outcome measures (and their categorization) is presented in Table 1.
Study design
The majority of studies examining dysphagia after ACDF surgery were retrospective (177/280 = 63%). The distribution of outcome measure by study design is presented in Fig. 2a and b.
Study year
The number of studies focusing on dysphagia after ACDF surgery has been steadily increasing over the past 20 + years. Figure 3 collates the most frequent outcome measure types by 5-year age bins. Notably, validated dysphagia patient-reported outcome measures were first observed in the ACDF literature after 2011.
Study country
The majority of the studies were conducted in the USA (n = 143), followed by China (n = 74), Korea (n = 15), Japan (n = 7), Italy (n = 6), and Germany and Canada (n = 5). All other countries had fewer than five studies. The frequency ranking and order of top contributing countries did not change significantly when we restricted the dataset to only prospective studies.
Discussion
Dysphagia is the most common complication reported after ACDF surgery [10], yet the incidence is poorly understood. Dysphagia is a nuanced phenomenon and can be identified subjectively by the patient, quantified using validated scales, or confirmed using imaging techniques. We believe that the wide variety of methods used to identify dysphagia may be obscuring a more refined understanding of dysphagia (and risk of dysphagia) post-ACDF. Therefore, our goal was to conduct a scoping review to quantify the type of outcome measures used to identify dysphagia in the ACDF literature. Our scoping review identified 280 publications that reported dysphagia after ACDF.
As can be appreciated from Table 1, the greatest proportion of studies were classified as unvalidated patient-reported outcomes (nearly half of all outcome measures, 46%) which is largely driven by the popular use of the ‘Bazaz Scale’ [23]. This scale utilizes a numerical score to quantify difficulty swallowing ranging from 0 (none) to 3 (severe) for both liquids and solids. Very few methodological details are provided within the original study for how and who administers the questionnaire and why the levels of impairment are not congruent for liquids and solids. Despite its pervasive use in ACDF studies, this scale has never been validated. Indeed, recent studies have confirmed shortcomings of this scale. For example, Bazaz scores do not correlate with the MD Anderson Dysphagia Inventory (MDADI)—a validated dysphagia questionnaire for head and neck cancer [20], the Bazaz scale failed to capture dysphagia in 32% of cases when compared to the validated Eating Assessment Tool (EAT-10) [24], and the Bazaz scale has significant floor and ceiling effects compared to the Hospital for Special Surgery—Dysphagia and Dysphonia Index (HSS-DDI)—a validated patient-reported outcome measure designed specifically for the ACDF population [19].
The resulting situation is that studies using the Bazaz scale may limit what is understood about dysphagia after ACDF. Large-scale, longitudinal analyses have used this scale to track the rate and presentation of dysphagia over time (despite problematic floor and ceiling effects) as the primary outcome measure for dysphagia [23, 25, 26]. Further, studies investigating innovative interventions and techniques designed to mitigate dysphagia after ACDF have relied on the Bazaz scale such as the use of intraoperative steriods [27], instrumentation type [28], and the use of pretracheal retraction exercises [29].
The next highest proportion of outcome measure type was equal (16%) between two categories—nsufficient detail and validated patient-reported outcome measures/screening tools. Our study raters were instructed to choose insufficient detail when no description was provided for how dysphagia was captured in the methods section. In these studies, authors commonly report the incidence of dysphagia in their study within the results section, with no prior details on how this was captured. Replication of these studies would not be possible with respect to dysphagia incidence. Presumably, these data rely on surgeon records and/or surgeon recall. Unfortunately, research has shown that there is poor correlation between surgeon records and patient-reported data from structured questionnaires. Edwards and colleagues documented use of surgeon records caused under-reporting of dysphagia in 80% of cases [30].
Validated patient-reported outcome measures also accounted for 16% of the studies overall. These most commonly included the Eating Assessment Tool (EAT-10) [31] and the Swallowing Quality of Life Scale (Swal-QOL) [32] both of which are designed to be used with heterogeneous patient populations. We are encouraged to see increasing uptake of new screening tools that are psychometrically appropriate for ACDF populations, including the Dysphagia Short Questionnaire (DSQ) [33] and the Hospital for Special Surgery—Dysphagia and Dysphonia Index (HSS-DDI) [34]. As noted in Fig.3, the use of validated dysphagia metrics first appeared in 2011 and has positively and rapidly increased in the past decade.
Chart review/database outcome measures represent 13% of the overall data (exclusively in retrospective studies). This category and design of study is inherently limited (not only in ACDF) in the type of data that is gleaned; however, these studies typically yield important demographic and procedural information quantified from an impressive sample size.
Instrumental assessment accounts for 7% of all the studies that report dysphagia after ACDF surgery. Several trends emerge when we closely compiled the results of the instrumental studies in this scoping review. It appears that ACDF causes biomechanical disruptions to pharyngeal constriction/stripping [11, 35,36,37,38], hyoid bone excursion [36, 39, 40], epiglottic deflection [11, 37, 38], and upper esophageal sphincter opening [11, 36, 38, 41]. These biomechanical deficits largely explain the functional consequences observed on instrumental studies, most notably impaired swallowing efficiency (post-swallow residue) [36,37,38, 40, 42, 43]. Aspiration has also been documented in this population, but primarily of post-swallow residue [11, 36, 41] and/or in the acute phase [44]. VFSS has also been used to document significant pre-vertebral swelling post-ACDF [11, 36, 39, 40, 42, 44]; though, this can also be easily captured from routine lateral view radiographs.
Finally, our scoping review reveals that diet-based outcomes and clinical bedside evaluations each only represent approximately 1% of all studies that report dysphagia after ACDF.
We acknowledge several limitations of this scoping review. First, the use of a scoping review methodology meant that we did not appraise the quality of individual studies that we located. Certainly, this scoping review sets the stage for a future systematic review on this topic which would necessitate the systematic appraisal of individual study design, risk of bias and scientific rigor. Second, our review was limited to one reviewer per study, a necessary methodological decision given the sheer number of studies included; however, our excellent intra-rater agreement levels (≥ 90%) minimize this limitation. Finally, our review did not gather information about dysphagia incidence which would represent an interesting follow-up analysis of the studies we have compiled. If we were to examine the incidence of dysphagia by outcome measure type, we would also need to control for timing of assessment given that dysphagia often resolves in the early post-operative period. However, to our knowledge, the evolution/resolution of dysphagia has not been prospectively analyzed using instrumental swallowing assessment and therefore represents an important area for future research.
Recommendations for the future
Based on the results of this review, we advocate for a paradigm shift in the methods used to capture dysphagia after ACDF surgery. Above all else, given the significant rates of dysphagia after ACDF surgery, we strongly advocate for collaborative clinical and research partnerships between spine surgery teams and swallowing specialists such as otolaryngologists and speech language pathologists. A post hoc review of our data revealed that only 27 of the 280 publications that examined dysphagia after ACDF included a swallowing specialist as a co-author. Next, we recommend that surgical teams discontinue the use of the Bazaz scale for screening swallowing function given the limitations discussed above [19, 24, 33]. Instead, we advocate for universal screening of swallowing at post-surgical follow-up appointments using a validated patient-reported outcome measure. The 31-item HSS-DDI [34] makes an excellent candidate for screening given that it is validated specifically for ACDF patients and provides critical information about both dysphagia and dysphonia. Scores on the HSS-DDI range from 0 to 100 (lower scores represent better function) and a > 10-point reduction represents a clinically meaningful difference [45]. The EAT-10 [31] also is a strong candidate for universal screening given that it is very quick to administer (10 items) and has proposed cut points for referral for in-depth swallowing evaluation (scores ≥ 3). Early post-operative EAT-10 scores have been shown to predict long-term dysphagia symptoms in ACDF patients [46]. Then, these screening results can yield efficient and accurate referrals for instrumental swallowing assessments which we recognize are costly, time intensive, and require specialized staff and equipment. However, referrals based on universal screening at postoperative follow-up appointments should minimize the debilitating impact that dysphagia can have on health and quality of life for ACDF patients and will ensure that patients do not fall through the cracks only to end up in outpatient swallowing clinics several months later. Finally, when feasible, we strongly encourage future ACDF research designs to employ gold-standard VFSS assessments pre- and post-surgery. Expert analysis of these studies can yield nuanced continuous data on swallowing biomechanics that is likely to be sensitive to subtle differences between groups in studies aimed to reduce dysphagia post-ACDF.
Conclusion
This scoping review has revealed that the majority of studies reporting dysphagia after ACDF surgery rely on inappropriate outcome measures. Specifically, these account for nearly two-thirds of the literature we located—with unvalidated scales making up 46% and those that lack clear methodological details regarding dysphagia diagnosis representing an additional 16%. By and far, the most pervasive tool contributing to this is the Bazaz scale. At the same time, the use of validated patient-reported outcome measures for capturing dysphagia appears to be on the rise and new, validated, ACDF-specific metrics are available for immediate use. We advocate for the inclusion of swallowing specialists (otolaryngologists and/or speech language pathologists) in both the clinical management and research of ACDF patients. Universal screening at postoperative follow-up appointments using validated patient-reported metrics will improve identification of patients at risk for significant dysphagia. This will allow for efficient and timely referrals for in-depth swallowing evaluations using instrumental imaging of swallowing and in turn minimize the debilitating effects of dysphagia on health and quality of life.
References
Shaw SM, Martino R (2013) The normal swallow: muscular and neurophysiological control. Dysphagia Diagn Manage 46(6):937–956.
Schmidt J, Holas M, Halvorson K, Reding M (1994) Videofluoroscopic evidence of aspiration predicts pneumonia and death but not dehydration following stroke. Dysphagia 9(1):7–11
Serra-Prat M, Palomera M, Gomez C, Sar-Shalom D, Saiz A, Montoya JG et al (2012) Oropharyngeal dysphagia as a risk factor for malnutrition and lower respiratory tract infection in independently living older persons: a population-based prospective study. Age Ageing 41(3):376–381
Finestone HM, Foley NC, Woodbury MG, Greene-Finestone L (2001) Quantifying fluid intake in dysphagic stroke patients: a preliminary comparison of oral and nonoral strategies. ArchPhysMedRehabil 82(12):1744–1746
Chen PH, Golub JS, Hapner ER, Johns MM III (2009) Prevalence of perceived dysphagia and quality-of-life impairment in a geriatric population. Dysphagia 24(1):1–6
Bonilha HS, Simpson AN, Ellis C, Mauldin P, Martin-Harris B, Simpson K (2014) The one-year attributable cost of post-stroke dysphagia. Dysphagia 29(5):545–552.
Patel D, Krishnaswami S, Steger E, Conover E, Vaezi M, Ciucci M et al (2017) Economic and survival burden of dysphagia among inpatients in the United States. Dis Esophagus 31(1):dox131.
Attrill S, White S, Murray J, Hammond S, Doeltgen S (2018) Impact of oropharyngeal dysphagia on healthcare cost and length of stay in hospital: a systematic review. BMC Health Serv Res 18(1):594
Shune SE, Namasivayam-MacDonald A. (2020). Dysphagia-related caregiver burden: moving beyond the physiological impairment. Perspect ASHA SIGs 23(5):1282–1289.
Epstein NE (2019) A review of complication rates for anterior cervical diskectomy and fusion (ACDF). Surg Neurol Int 7(10):100
Leonard R, Belafsky P (2011) Dysphagia following cervical spine surgery with anterior instrumentation: evidence from fluoroscopic swallow studies. Spine 36(25):2217–2223
Yerneni K, Burke JF, Nichols N, Tan LA (2019) Delayed recurrent laryngeal nerve palsy following anterior cervical discectomy and fusion. World Neurosurg 122:380–383
Skoretz SA, Flowers HL, Martino R (2010) The incidence of dysphagia following endotracheal intubation a systematic review. Chest 137(3):665–673
Haller JM, Iwanik M, Shen FH (2011) Clinically relevant anatomy of high anterior cervical approach. Spine 36(25):2116–2121
Haller JM, Iwanik M, Shen FH (2012) Clinically relevant anatomy of recurrent laryngeal nerve. Spine 37(2):97–100
Shriver MF, Lewis DJ, Kshettry VR, Rosenbaum BP, Benzel EC, Mroz TE (2017) Dysphagia rates after anterior cervical diskectomy and fusion: a systematic review and meta-analysis. Global Spine J 7(1):95–103
Yee TJ, Swong K, Park P (2020) Complications of anterior cervical spine surgery: a systematic review of the literature. J Spine Surg 6(1):302–322
Rosenthal BD, Nair R, Hsu WK, Patel AA, Savage JW (2016) Dysphagia and dysphonia assessment tools after anterior cervical spine surgery. Clin Spine Surg 29(9):363–367
Liang G, Zheng X, Liang C, Chen C, Huang Y, Huang S et al (2022) Comparison of Bazaz scale, dysphagia short questionnaire, and hospital for special surgery-dysphagia and dysphonia inventory for assessing dysphagia symptoms after anterior cervical spine surgery in Chinese Population. Dysphagia 37(2):250–259
Skeppholm M, Ingebro C, Engström T, Olerud C (2012) The dysphagia short questionnaire: an instrument for evaluation of dysphagia. Spine 37(11):996–1002
Martin-Harris B, Brodsky MB, Michel Y, Castell DO, Schleicher M, Sandidge J et al (2008) MBS measurement tool for swallow impairment-MBSimp: establishing a standard. Dysphagia 23(4):392–405
Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E (2018) Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol 18(1):143
Bazaz R, Lee MJ, Yoo JU (2002) Incidence of dysphagia after anterior cervical spine surgery: a prospective study. Spine 27(22):2453–2458
Rosenthal BD, McCarthy MH, Bhatt S, Savage JW, Singh K, Hsu WK et al (2019) A comparison of patient-centered outcome measures to evaluate dysphagia and dysphonia after anterior cervical discectomy and fusion. JAAOS J Am Acad Orthopaedic Surg 27(22):848–853
Lee MJ, Bazaz R, Furey CG, Yoo J (2007) Risk factors for dysphagia after anterior cervical spine surgery: a two-year prospective cohort study. Spine Journal 7(2):141–147
Lee MJ, Bazaz R, Furey CG, Yoo J (2005) Influence of anterior cervical plate design on dysphagia: a 2-year prospective longitudinal follow-up study. J Spinal Disord Tech 18(5):406–409
Edwards CC, Dean C, Phillips D, Blight A (2016) Can dysphagia following anterior cervical fusions with rhBMP-2 be reduced with local depomedrol application?: a prospective, randomized, placebo-controlled, double-blind trial. Spine 41(7):555–562
Liu Y, Wang H, Li X, Chen J, Sun H, Wang G et al (2016) Comparison of a zero-profile anchored spacer (ROI-C) and the polyetheretherketone (PEEK) cages with an anterior plate in anterior cervical discectomy and fusion for multilevel cervical spondylotic myelopathy. Eur Spine J 25(6):1881–1890
Chaudhary SK, Yu B, Pan F, Li X, Wang S, Shaikh II et al (2017) Manual preoperative tracheal retraction exercise decreases the occurrence of postoperative oropharyngeal dysphagia after anterior cervical discectomy and fusion. J Orthopaedic Surg 25(3).
Edwards CC II, Karpitskaya Y, Cha C, Heller JG, Lauryssen C, Yoon ST et al (2004) Accurate identification of adverse outcomes after cervical spine surgery. J Bone Joint Surg Ser A 86(2):251–256
Belafsky PC, Mouadeb DA, Rees CJ, Pryor JC, Postma GN, Allen J et al (2008) Validity and reliability of the eating assessment tool (EAT-10). Ann Otol Rhinol Laryngol 117(12):919–924
McHorney CA, Robbins J, Lomax K, Rosenbek JC, Chignell K, Kramer AE et al (2002) The SWAL-QOL and SWAL-CARE outcomes tool for oropharyngeal dysphagia in adults: III Documentation of reliability and validity. Dysphagia 17(2):97–114
Skeppholm M, Ingebro C, Engström T, Olerud C (2012) The dysphagia short questionnaire: an instrument for evaluation of dysphagia: a validation study with 12 months’ follow-up after anterior cervical spine surgery. Spine 37(11):996–1002
Hughes AP, Salzmann SN, Aguwa OK, Miller CO, Duculan R, Shue J et al (2018) HSS Dysphagia and Dysphonia Inventory (HSS-DDI) following anterior cervical fusion: patient-Derived, validated, condition-specific patient-reported outcome measure outperforms existing indices. J Bone Joint Surg Am 100(10):e66
Ian Dhar S, Wegner AM, Rodnoi P, Wuellner JC, Mehdizadeh OB, Shen SC, et al (2020) Fluoroscopic swallowing abnormalities in dysphagic patients following anterior cervical spine surgery. Ann Otol Rhinol Laryngol 129(11):1101–1109.
Miles A, Jamieson G, Shasha L, Davis K (2021) Characterizing dysphagia after spinal surgery. J Spinal Cord Med 44(5):733–741.
Martin RE, Neary MA, Diamant NE (1997) Dysphagia following anterior cervical spine surgery. Dysphagia 12(1):2–10
Carucci LR, Turner MA, Yeatman CF (2015) Dysphagia secondary to anterior cervical fusion: radiologic evaluation and findings in 74 patients. AmJRoentgenol 204(4):768–775
Kang SH, Kim DK, Seo KM, Lee SY, Park SW, Kim Y (2016) Swallowing function defined by videofluoroscopic swallowing studies after anterior cervical discectomy and fusion: a prospective study. J Korean Med Sci 31(12):2020–2025
Muss L, Wilmskoetter J, Richter K, Fix C, Stanschus S, Pitzen T et al (2017) Changes in swallowing after anterior cervical discectomy and fusion with instrumentation: a presurgical versus postsurgical videofluoroscopic comparison. J Speech Lang Hear Res 60(4):785–793
Smith-Hammond CA, New KC, Pietrobon R, Curtis DJ, Scharver CH, Turner DA (2004) Prospective analysis of incidence and risk factors of dysphagia in spine surgery patients: Comparison of anterior cervical, posterior cervical, and lumbar procedures. Spine 29(13):1441–1446
Frempong-Boadu A, Houten JK, Osborn B, Opulencia J, Kells L, Guida DD et al (2002) Swallowing and speech dysfunction in patients undergoing anterior cervical discectomy and fusion: a prospective, objective preoperative and postoperative assessment. J Spinal Disord Tech 15(5):362–368
Min Y, Kim WS, Kang SS, Choi JM, Yeom JS, Paik NJ (2016) Incidence of dysphagia and serial videofluoroscopic swallow study findings after anterior cervical discectomy and fusion a prospective study. Clin Spine Surg 29(4):E177–E181
Ziegler JP, Davidson K, Cooper RL, Garand KL, Nguyen SA, Yuen E et al (2021) Characterization of dysphagia following anterior cervical spine surgery. Adv Commun Swallow 24(1):55–62
Okano I, Ortiz Miller C, Salzmann SN, Hoshino Y, Shue J, Sama AA et al (2020) Minimum clinically important differences of the hospital for special surgery dysphagia and dysphonia inventory and other dysphagia measurements in patients undergoing ACDF. Clin Orthop Relat Res 478(10):2309–2320
Haller L, Mehul Kharidia K, Bertelsen C, Wang J, O’Dell K (2022) Post-operative dysphagia in anterior cervical discectomy and fusion. Ann Otol Rhinol Laryngol 131(3):289–294
Acknowledgements
The authors would like to thank Marian Isdahl, Julie Bancroft, and Emely Dominguez for their assistance with this scoping review. This research was supported in part by pilot funds from the NIH/NCATS UL1TR001445 awarded to Molfenter, Balou, Amin & Frempong-Boadu.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Molfenter, S.M., Amin, M.R., Balou, M. et al. A scoping review of the methods used to capture dysphagia after anterior cervical discectomy and fusion: the need for a paradigm shift. Eur Spine J 32, 969–976 (2023). https://doi.org/10.1007/s00586-022-07515-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-022-07515-1