Abstract
Purpose
This study aimed to evaluate the mid-term efficacy and safety of Escherichia coli-derived bone morphogenetic protein-2 (E.BMP-2)/hydroxyapatite (HA) in lumbar posterolateral fusion (PLF).
Methods
This multicenter, evaluator-blinded, observational study utilized prospectively collected clinical data. We enrolled 74 patients who underwent lumbar PLF and had previously participated in the BA06-CP01 clinical study, which compared the short-term outcomes of E.BMP-2 with an auto-iliac bone graft (AIBG). Radiographs and CT scans were analyzed to evaluate fusion grade at 12, 24, and 36 months. Visual analog scale (VAS), Oswestry disability index (ODI), and Short Form-36 (SF-36) scores were measured preoperatively and at 36 months after surgery. All adverse events in this study were assessed for its relationship with E.BMP-2.
Results
The fusion grade of the E.BMP-2 group (4.91 ± 0.41) was superior to that of the AIBG group (4.25 ± 1.26) in CT scans at 36 months after surgery (p = 0.007). Non-union cases were 4.3% in the E.BMP-2 and 16.7% in the AIBG. Both groups showed improvement in pain VAS, ODI, and SF-36 scores when compared to the baseline values, and there were no statistically significant differences between the two groups. No treatment-related serious adverse reactions were observed in either group. No neoplasm-related adverse events occurred in the E.BMP-2 group.
Conclusions
The fusion quality of E.BMP-2/HA was superior to that of AIBG. E.BMP-2/HA showed comparable mid-term outcomes to that of AIBG in terms of efficacy and safety in one-level lumbar PLF surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In lumbar fusion surgery, achieving solid bone fusion is essential to accomplish good clinical outcomes because it affects postoperative functional outcome, low back pain and postoperative radiating pain [1, 2]. However, fusion rate of posterior lumbar fusion has stagnated to 88–95%, and approximately 10% of patients still experience nonunion [3].
Although autologous iliac bone graft (AIBG) is the gold standard, approximately 18% of patients reported graft site pain or discomfort 2 years after surgery [4]. In an effort to enhance the fusion process, application of recombinant human bone morphogenetic protein-2 (rhBMP-2) resulted in satisfactory fusion rates in anterior lumbar interbody fusion and posterolateral fusion (PLF) [4,5,6].
Currently, most rhBMP-2 is being produced in Chinese hamster ovary (CHO) cells which are mammalian cells. As CHO cell BMP-2 (C.BMP-2) is produced by purifying the secreted product through post-translational modification of mammalian cell, its production efficacy is very low, making mass production difficult [7, 8]. To overcome these shortcomings, Escherichia coli-derived rhBMP-2 (E.BMP-2) has been introduced as a new source of rhBMP-2 [9, 10]. Several in vitro and in vivo studies have shown that the biological activity of E.BMP-2 is comparable to that of C.BMP-2 [8, 11,12,13]. Previously the authors reported the efficacy and safety outcomes of E.BMP-2 with hydroxyapatite (Ca10(PO4)6(OH)2, HA) [14] carrier in single-level PLF and the result was comparable to that of AIBG at 6 months. [15] However, the follow-up period was a short time to confirm the safety of the graft. Also, there was limitation to previous study because the fusion evaluation was done before HA carrier was absorbed. Furthermore, follow-up studies for more than 3 years after using E.BMP-2 in lumbar PLF have not been reported.
Therefore, this study aimed to report the mid-term efficacy and safety outcomes of E.BMP-2/HA compared with those of AIBG in patients who underwent 1 level PLF.
Materials and methods
Study design
The present study is a multicenter, evaluator-blinded, prospective, and retrospective observational study that aims to establish the mid-term safety and efficacy in patients who had received E.BMP-2/HA or AIBG through lumbar PLF in a previously performed clinical trial(BA06-CP01) [15]. Among the subjects who completed the previous study conducted from March 2013 to 2016, patients who were followed-up for > 36 months with available efficacy data were enrolled and analyzed.
The inclusion criteria of the BA06-CP01trial included patients ≤ 80 years old and who required 1-level posterior lumbar fusion because of spinal stenosis, grade 1 spondylolisthesis or spondylolysis. The exclusion criteria were as follows: average spine T-score < − 3.0 on dual-energy X-ray absorptiometry, history of cancer, hypocalcemia, and specific conditions with internal, endocrine, or psychiatric disorders that makes lumbar fusion difficult or impossible. [15]
Basic information, medical history/surgical history, clinical questionnaire results (Oswestry disability index [ODI], SF-36, and VAS), radiological outcomes (CT and X-ray), group allocation information, and ongoing adverse events were collected before surgery from the BA06-CP01 clinical trial. Hospital records of subjects were retrospectively collected at 12, 24, and 36 months. If patients in the BA06 CP01 clinical trial voluntarily visited the hospital after 36 months, written consent was obtained and they were included as participants. Retrospective study data were collected, and prospective visiting participants were recruited until July 31, 2020. This study was approved by the institutional review board of each institution and was conducted according to the Declaration of Helsinki and the guidelines for Good Clinical Practice.
Intervention
Lumbar PLF was performed routinely. Following the posterior midline approach, decompression with laminectomy and flavectomy was performed. Pedicle screw fixation was performed at the involved level and the allocated bone graft materials were applied between the two transverse processes [15]. In the E.BMP-2 group, we used Novosis (CG Bio Co., Ltd., Seoul, Korea), an E. coli-derived rhBMP-2 with an HA carrier. Approximately 3 g (8 cc) of HA was soaked in one vial (3.0 mg) of E.BMP-2 and carefully applied in the inter-transverse space. This process was repeated on the contralateral side. In the AIBG group, approximately 8 cc of auto-iliac bone graft was applied on each side. Bone grafts obtained via laminectomy were not used in either group.
Efficacy evaluation
Radiologic outcome
The fusion grade was mainly assessed by bone bridging in coronal reconstruction images of 36-month three-dimensional CT (3D CT, thin cut, 2 mm or less interval) scans. The fusion grade was additionally and independently evaluated by radiographs at 12/24/36 months. The fusion status was assessed by two blinded independent orthopedic surgeons who did not participate in the clinical trial. The fusion grade was defined as follows: grade 1, no fusion; grade 2, partial or limited unilateral; grade 3, partial or limited bilateral; grade 4, solid unilateral; and grade 5, solid bilateral [16]. To ensure stricter fusion rate evaluation than in the previous study, only fusion grades 4 and 5 were defined as successful fusion. If the evaluators’ opinions differed, a final consensus was obtained. The width of the fusion mass was measured using a CT scan at ≥ 36 months, which was then summed and recorded. To evaluate the maturation of the fusion mass, trabeculation of the fusion mass was evaluated. Ectopic bone formation near the intervertebral foramen was also evaluated.
Clinical outcome
Pain VAS (lower back, right leg, and left leg), ODI and SF-36 scores were evaluated preoperatively and at 36 months postoperatively. Changes in the ODI, SF-36 and VAS scores at ≥ 36 months from baseline were assessed. Moreover, the incidence rate of additional interventional surgeries was also recorded.
Safety evaluation
All adverse events were standardized as “System Organ Class” and “Preferred Term” using MedDRA 23.0. [17] Adverse events (subjective or objective symptoms) occurring after surgery were evaluated at each follow-up visit of each body part. Moreover, the relationship between each event was assessed for its relationship with E.BMP-2. Death, life threat, prolonged hospitalization, severe disability, or continued incapacity were assessed as severe complications.
Statistical analysis
Statistical analyses were performed using the Statistical Analysis System (SAS Institute Inc.), and two-sided tests were performed at a significance level of 0.05. Comparative analysis between the groups was performed using the two-sample t-test or Wilcoxon rank-sum test for continuous variables to determine whether the data were normally distributed. The chi-square test or Fisher’s exact test was performed for categorical variables. Differences between groups in percent change in ODI, SF-36 and VAS scores at ≥ 36 months compared with those at baseline were analyzed using analysis of covariance.
Results
Study population and demographic data
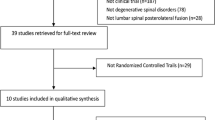
Of the 78 patients who completed the 6-month postoperative visit in the previous BA06-CP01 clinical trial, 74 patients (94%) were enrolled in this study (32 patients in the E.BMP-2 group and 42 patients in the AIBG group), and four patients were excluded because of loss of follow-up. Seventy-three patients were included in the efficacy analysis, and one patient with no efficacy results was excluded. All 74 patients were included in the safety evaluation (Fig. 1). The average ages of the E.BMP-2 and AIBG groups were 63.19 (± 8.18) and 60.83 (± 8.95) years, respectively, and there were no significant differences between the two groups in age, sex ratio, height, weight, body mass index, smoking history, drinking history, BMD, and preoperative radiologic findings (all p > 0.05) (Table 1).
Efficacy outcomes
-
(1)
Radiologic outcome
The CT-based bone fusion grade was 4.91 (± 0.42) in the E.BMP-2 group and 4.25 (± 1.26) in the AIBG group at 36 months, which was significantly higher in the E.BMP-2 group (p = 0.007). The bone fusion rates of the E.BMP-2 and AIBG groups over 36 months were 95.7% (22/23) and 83.3% (20/24), respectively; however, there was no significant difference between the two groups (p = 0.348). Moreover, the proportion of grade 5 fusion was higher in the E.BMP-2 than in the AIBG group (96.6% vs. 62.5%). The fusion mass in the 36-month CT scan was significantly wider in the E.BMP-2 group (31.1 mm) than in the AIBG group (22.9 mm) (p = 0.006), and trabeculation of the fusion mass was 100% in both groups (Table 2, Fig. 2). The plain radiograph-based fusion grades at 12 and ≥ 36 months were significantly higher in the E.BMP-2 group than in the AIBG group (p < 0.05, Table 3). No ectopic bone formation was observed at the intervertebral foramen which could cause radiculopathy.
-
(2)
Clinical outcome
Characteristics of the fusion mass taken at 6 and ≥ 36 months after surgery fusion mass of E.BMP-2 group (A-D) and AIBG group (E-H) taken by X-ray or CT at each time point. The remaining HA carrier granules of the E.BMP-2 group at 6 months (B) became trabeculated mature bones at > 36 months (D). At ≥ 36 months, the width of the fusion mass in the E.BMP-2 group (D) was more expansive than in the AIBG group (H). AIBG, autogenous iliac bone graft; E.BMP-2, Escherichia coli-derived recombinant human bone morphogenetic protein-2; HA, hydroxyapatite.
The clinical parameters showed postoperative improvement in both groups. The changes in the VAS (lumbar, right leg, and left leg), ODI, and SF-36 scores at ≥ 36 months are shown in Table 4. There was no significant difference between the two groups in terms of VAS scores for lumbar and leg pain (lumbar p = 0.309; left side, p = 0.1456; right side, p = 0.9463). In both groups, the ODI score decreased at 36 months after surgery, and the SF-36 score improved compared to that at baseline, but there was no significant difference between the two groups (ODI, p = 0.4105; SF-36, p = 0.7229) (Tables 4, 5). No additional re-operation was performed at the surgical site during the follow-up period in either group.
Safety outcome
The most common adverse reaction after surgery was back pain in both groups which accounted for 18 patients (56.25%, 20 cases) in the E.BMP-2 group and 16 patients (38.10%, 19 cases) in the AIBG group. The incidence of adverse reactions was not significantly different between the two groups (p = 0.5277, Supplementary Table 1). Serious treatment-related adverse events occurred in five patients (15.63%, 11 cases) in the E.BMP-2 group and in eight patients (19.05%, 10 cases) in the AIBG group, but the difference in incidence between the groups was not statistically significant (p = 0.7015, Tables 4, 5). One patient in the AIBG group was diagnosed with lung adenocarcinoma; however, no occurrences of cancer were observed in the E.BMP-2 group (Table 4, 5).
Discussion
Unlike C.BMP-2, which has been used for almost two decades, reports on efficacy and safety of E.BMP-2 for spine fusion are deficient. To our knowledge, clinical reports of E.BMP-2 for over 3 years are absent. Moreover, the fate of the HA granule identified at 6 months of E.BMP-2/HA has not been elucidated. Therefore, we attempted to evaluate mid-term efficacy and safety by analyzing the 3-year follow-up results of patients treated with E.BMP-2. At 36 months postoperatively, the fusion grade of the E.BMP-2 group was significantly higher than that of the AIBG group (E.BMP-2, 4.91 vs. AIBG, 4.25); however, there was no significant difference in fusion rate, clinical and safety outcomes. In the present study, stricter criteria (grades 4 and 5) were applied than those used in the BA06-CP01 study (grade 2, 3, 4, and 5) to confirm fusion. For this reason, it seems that the fusion rate of both groups was lower than those observed in the previous BA06-CP01 study (100.0% and 90.2%) [15] even with a longer follow-up period. However, using the same fusion criteria, the fusion rates in this study would be 100% and 91.6%, which are consistent with the previous study.
Considering the higher ratio of the fusion grade 5 and wider fusion mass in the BMP-2 group, the fusion quality of the BMP-2 group was considered to be higher than the AIBG group. An important finding of this study was that a larger fusion mass was formed even with the same volume of graft inserted. Sheehan et al. also reported that the fusion volume increased compared to AIBG when CHO-BMP-2/collagen was used in a canine model [18]. Therefore, we suggest that the E.BMP-2 with HA forms early but immature spinal fusion at 6 months, and then the HA granules are converted into a solid fusion mass over time without reduction in volume (Fig. 2).
C.BMP-2 has been reported to have good clinical outcomes in spinal surgery. [19] Achieving an early solid bone fusion can provide patients with faster postoperative recovery and lower reoperation rates [20]. Dimar et al. reported good results not only in ODI but also in functional aspects such as, return to work, when using BMP-2 [21]. However, it has been reported that CHO cell-derived BMP-2 (C.BMP-2) can increase the cost of surgery by 10–14% compared to conventional surgery [20]. This is closely related to the production efficiency of BMP-2 and the dose of C.BMP-2 per fusion level; however, there is still no consensus on the ideal dose of BMP-2. An excessive BMP-2 dose not only increases cost but also causes osteolysis and increases the incidence of spinal tumors [22]; therefore, it is important to use the optimal dose of BMP-2. Son et al. reported a 100% fusion rate in addition to interbody fusion with a relatively small dose when applying 1 mg of BMP-2 to PLF. Slosar et al. reported 100% fusion rates for 3 mg/level of C.BMP-2 combined with an allogenous bone graft in anterior lumbar fusion. In this study, 6 mg/level of E.BMP-2/HA was sufficient for solid fusion in PLF without ectopic bone formation or tumor-related event.
BMP-2 is a water-soluble protein that inherently requires a carrier. An ideal carrier should effectively absorb BMP-2, allow slow controlled release of BMP-2, provide scaffold, and should gradually degrade. In bio-ceramics such as HA or tricalcium phosphate, rhBMP-2 is not only absorbed into the scaffold but is also adsorbed as a non-covalent bond on the HA surface. Because collagen carriers are degraded or absorbed within a few weeks in the human body, BMP-2 absorbed in collagen can only be scattered by collagen degradation [22]. In preclinical experiments using Rabbit and minipig, when HA was used as an E.BMP-2 carrier, it showed successful fusion in PLF [10, 12]. In this study, retention of BMP-2 was maintained by remaining on the HA granule surface for over 6 months, which may have a positive effect on fusion. Several studies have reported better results using a compression-resistant carrier with C.BMP-2 than with AIBG in posterolateral fusion, where mechanical muscle compression is expected [23], 24. In this respect, the HA carrier is considered as one of the appropriate carrier options in the lumbar PLF.
This study had several limitations. First, the number of patients in each group was relatively small (32 and 41, respectively). However, it was sufficient to compare fusion grade and fusion quality. Second, not all patients underwent CT scans which may have caused selection bias in the evaluation of fusion rate. Nevertheless, the result of this study is important as the first mid-term follow-up study using E.BMP-2 for lumbar fusion surgery.
Conclusion
In conclusion, E.BMP-2/HA showed a comparable efficacy and safety in the mid-term follow-up compared with AIBG in single-segment posterolateral fusion. E.BMP-2/HA can be a satisfactory alternative to AIBG in patients who require fast and solid fusion.
References
Kornblum MB, Fischgrund JS, Herkowitz HN, Abraham DA, Berkower DL, Ditkoff JS (2004) Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective long-term study comparing fusion and pseudarthrosis. Spine 29:726–733
Kim Y-H, Ha K-Y, Rhyu K-W, Park H-Y, Cho C-H, Kim H-C, Lee H-J, Kim S-I (2020) Lumbar interbody fusion: techniques pearls and pitfalls. Asian Spine J 14:730
Turner JA, Herron L, Deyo RA (1993) Meta-analysis of the results of lumbar spine fusion. Acta Orthop Scand 64:120–122
Goulet JA, Senunas LE, DeSilva GL, Greenfield MLV (1997) Autogenous iliac crest bone graft: complications and functional assessment. Clin Orthop Relat Res® 339:76–81
Burkus JK, Gornet MF, Dickman CA, Zdeblick TA (2002) Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. Clin Spine Surg 15:337–349
Galimberti F, Lubelski D, Healy AT, Wang T, Abdullah KG, Nowacki AS, Benzel EC, Mroz TE (2015) A systematic review of lumbar fusion rates with and without the use of rhBMP-2. Spine 40:1132–1139. https://doi.org/10.1097/brs.0000000000000971
Kim CL, Jung MY, Kim YS, Jang JW, Lee GM (2018) Improving the production of recombinant human bone morphogenetic protein-4 in Chinese hamster ovary cell cultures by inhibition of undesirable endocytosis. Biotechnol Bioeng 115:2565–2575. https://doi.org/10.1002/bit.26798
Jin Y-Z, Zheng G-B, Lee JH (2019) Escherichia coli BMP-2 showed comparable osteoinductivity with Chinese hamster ovary derived BMP-2 with demineralized bone matrix as carrier. Growth Factors 37:85–94
Lee JH, Jang S-J, Koo T-Y, Suh CW, Lee EN, Lee K-M, Lee HS, Baek H-R (2011) Expression, purification and osteogenic bioactivity of recombinant human BMP-2 derived by Escherichia coli. Tissue Eng Regen Med 8:8–15
Lee JH, Yu CH, Yang JJ, Baek H-R, Lee K-M, Koo T-Y, Chang B-S, Lee C-K (2012) Comparative study of fusion rate induced by different dosages of Escherichia coli–derived recombinant human bone morphogenetic protein-2 using hydroxyapatite carrier. Spine J 12:239–248
Kim IS, Lee EN, Cho TH, Song YM, Hwang SJ, Oh JH, Park EK, Koo TY, Seo YK (2011) Promising efficacy of Escherichia coli recombinant human bone morphogenetic protein-2 in collagen sponge for ectopic and orthotopic bone formation and comparison with mammalian cell recombinant human bone morphogenetic protein-2. Tissue Eng Part A 17:337–348. https://doi.org/10.1089/ten.TEA.2010.0408
Kong C-B, Lee JH, Baek H-R, Lee C-K, Chang B-S (2014) Posterolateral lumbar fusion using Escherichia coli–derived rhBMP-2/hydroxyapatite in the mini pig. Spine J 14:2959–2967
Jin Y-Z, Zheng G-B, Lee JH, Han S-H (2021) Comparison of demineralized bone matrix and hydroxyapatite as carriers of Escherichia coli recombinant human BMP-2. Biomater Res 25:1–7
Kim DK, Lee SJ, Cho TH, Hui P, Kwon MS, Hwang SJ (2010) Comparison of a synthetic bone substitute composed of carbonated apatite with an anorganic bovine xenograft in particulate forms in a canine maxillary augmentation model. Clin Oral Implant Res 21:1334–1344
Cho JH, Lee JH, Yeom JS, Chang B-S, Yang JJ, Koo KH, Hwang CJ, Lee KB, Kim H-J, Lee C-K (2017) Efficacy of Escherichia coli-derived recombinant human bone morphogenetic protein-2 in posterolateral lumbar fusion: an open, active-controlled, randomized, multicenter trial. Spine J 17:1866–1874
Glassman SD, Dimar JR, Carreon LY, Campbell MJ, Puno RM, Johnson JR (2005) Initial fusion rates with recombinant human bone morphogenetic protein-2/compression resistant matrix and a hydroxyapatite and tricalcium phosphate/collagen carrier in posterolateral spinal fusion. Spine 30:1694–1698
Son HJ, Choi SH, Lee MK, Kang C-N (2021) Efficacy and safety of Escherichia coli-derived recombinant human bone morphogenetic protein-2 in additional lumbar posterolateral fusion: minimum 1 year follow-up. Spine J 21(8):1340–1346
Sheehan JP, Kallmes DF, Sheehan JM, Jane JA, Fergus AH, DiPierro CG, Simmons NE, Makel DD, Helm GA (1996) Molecular methods of enhancing lumbar spine fusion. Neurosurgery 39:548–554
Burkus JK, Dorchak JD, Sanders DL (2003) Radiographic assessment of interbody fusion using recombinant human bone morphogenetic protein type 2. Spine 28:372–377
Cahill KS, Chi JH, Groff MW, McGuire K, Afendulis CC, Claus EB (2011) Outcomes for single-level lumbar fusion: the role of bone morphogenetic protein. Spine 36:2354
Dimar JR, Glassman SD, Burkus KJ, Carreon LY (2006) Clinical outcomes and fusion success at 2 years of single-level instrumented posterolateral fusions with recombinant human bone morphogenetic protein-2/compression resistant matrix versus iliac crest bone graft. Spine 31:2534–2539
DeVine JG, Dettori JR, France JC, Brodt E, McGuire RA (2012) The use of rhBMP in spine surgery: is there a cancer risk? Evid-based spine-care J 3:035–041
Dawson E, Bae HW, Burkus JK, Stambough JL, Glassman SD (2009) Recombinant human bone morphogenetic protein-2 on an absorbable collagen sponge with an osteoconductive bulking agent in posterolateral arthrodesis with instrumentation: a prospective randomized trial. JBJS 91:1604–1613
Boden SD, Martin GJ Jr, Morone MA, Ugbo JL, Moskovitz PA (1999) Posterolateral lumbar intertransverse process spine arthrodesis with recombinant human bone morphogenetic protein 2/hydroxyapatite–tricalcium phosphate after laminectomy in the nonhuman primate. Spine 24:1179–1185
Acknowledgements
This study was supported by a research grant for clinical studies from CGBio Inc/BioAlpha Inc (Gyeonggi-do, Korea).
Author information
Authors and Affiliations
Contributions
JHL conceptualized, designed, supervised the study, and contributed to data collection and analysis. MJC carried out data analysis and drafted the manuscript. KHY carried out data analysis, and JSY, HMK, KBL, JHC, and JJY collected data. All authors contributed to the manuscript revision, read, and approved the article's final version.
Corresponding author
Ethics declarations
Conflict of interests
MJC, KHY, JHC: Nothing to disclose. JHL, JSY, HMK, KBL, JHC, JJY: Grant:CG Bio/BioAlpha Inc (Paid directly to institution), pertaining to the submitted work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cho, M., You, KH., Yeom, J.S. et al. Mid-term efficacy and safety of Escherichia coli-derived rhBMP-2/hydroxyapatite carrier in lumbar posterolateral fusion: a randomized, multicenter study. Eur Spine J 32, 353–360 (2023). https://doi.org/10.1007/s00586-022-07440-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-022-07440-3