Abstract
Study design
Prospective multi-center study.
Objective
The study aimed to evaluate the accuracy of pedicle screw placement using a skin marker-based optical surgical navigation system for minimal invasive thoraco-lumbar-sacral pedicle screw placement.
Methods
The study was performed in a hybrid Operating Room with a video camera-based navigation system integrated in the imaging hardware. The patient was tracked with non-invasive skin markers while the instrument tracking was via an on-shaft optical marker pattern. The screw placement accuracy assessment was performed by three independent reviewers, using the Gertzbein grading. The screw placement time as well as the staff and patient radiation doses was also measured.
Results
In total, 211 screws in 39 patients were analyzed for screw placement accuracy. Of these 32.7% were in the thoracic region, 59.7% were in the lumbar region, and 7.6% were in the sacral region. An overall accuracy of 98.1% was achieved. No screws were deemed severely misplaced (Gertzbein grading 3). The average time for screw placement was 6 min and 25 secs (± 3 min 33 secs). The average operator radiation dose per subject was 40.3 µSv. The mean patient effective dose (ED) was 11.94 mSv.
Conclusion
Skin marker-based ON can be used to achieve very accurate thoracolumbarsacral pedicle screw placements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In minimally invasive spine surgery (MISS), the use of intra-operative fluoroscopy can mitigate issues associated with the loss of direct visualization of anatomic landmarks. However, only relying on fluoroscopy in MISS [1] is associated with pedicle perforation rates in the range of 12.5–13.5%. Computer-assisted navigation (CAN) offers the promise of improving screw placement accuracy [2,3,4, 6, 7], reducing radiation exposure, and reducing complications during MISS procedures [2, 30].
Currently available CAN platforms rely on infra-red camera-based tracking and bone-anchored dynamic reference frames (DRF) [8]. The infra-red cameras have the disadvantage of requiring a clear line- of-sight. Any movement of the DRF and the distance of the operating level from the DRF may influence accuracy [9].
A video camera-based optical navigation system (ClarifEye, Philips) was recently developed. Optical Navigation (ON) relies on skin markers in place of invasive DRFs for patient tacking and on-shaft optical markers for live instrument tracking. This system was previously shown to have high accuracy in complex open deformity cases [10] and a pre-clinical study reported on the accuracy of the system for percutaneous pedicle screw placements in the thoracolumbarsacral spine compared with fluoroscopy [11].
The study aimed to evaluate the accuracy of pedicle screw placement using a skin marker-based optical surgical navigation system for minimal invasive thoraco-lumbar-sacral pedicle screw placement. Here, we present the first clinical results on screw placement accuracy using this system. Secondary objectives include procedure time and the patient and staff radiation dose.
Materials and methods
This was a prospective study with a primary objective of collecting accuracy data on pedicle screw placements when using the ON system. The study was approved by the ethics committee and competent authorities in the involved countries (Germany: Medizinische Fakultat Der Christian-Albrechts-Universitat Zu Kiel ethic committee (00,011,811) and BfArM (00,011,828) and Switzerland: Comitato Etico del Cantone Ticino (2019–00,378) and Swissmedic (10,000,492)). Only subjects older than 18 years and undergoing percutaneous spine surgery in the thoracolumbar sacral spine in a maximum of 4 levels, were enrolled. All enrolled patients provided the informed consent.
The study was performed in a hybrid Operating Room equipped with a radiolucent, motorized, carbon fiber surgical table. 3D Cone Beam-Computed Tomography (CBCT) (XperCT, Philips, Best, the Netherlands) scans were done intra-operatively using a robotic ceiling-mounted C-arm system (AlluraClarity Flexmove, Philips, Best, The Netherlands). Navigation guidance was provided by ON (ClarifEye, Philips), based on video inputs from four optical cameras, rigidly integrated on each side of the detector frame (Fig. 1).
Devices and software used as a part of the study. Imaging was provided by the Allura angiographic system. The cameras in the detector (marked with a blue circle) provided the video input of the surgical site. The augmented reality surgical navigation (ARSN) system software was used to provide navigational guidance. The adhesive skin markers are used to track the patient movements. The Jamshidi needle had an optical pattern engraved on the shaft, which was used by the video cameras to provide 3D tracking of the needle in relation to the 3D scan
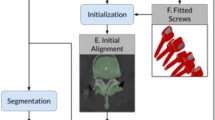
Surgery was performed under general anesthesia with patient in prone position. Approximately 8–10 optical skin markers (Philips, The Netherlands) were attached to the patient’s back, in a random pattern, around the surgical site (Fig. 2). A 10 s 3D CBCT was performed by robotic rotation of the C-arm through 180 ̊and an automatic 3D segmentation was obtained. The segmented view of the spinal volume with the vertebra labeled and pedicles highlighted, together with the multiplanar slice display was shown on a medical grade monitor (Fig. 2). The surgeon selected the desired pedicles and manually adjusted the trajectory, and dimensions of the virtually placed screw. A choice of four C-arm positions was provided to the surgeon, based on which of the four cameras was aligned to the planned trajectory. The surgeon chose the C-arm position which provided the best working position.
Workflow description of augmented reality surgical navigation (ARSN) system during the clinical study. The steps of the workflow are shown sequentially and involves: Attaching the skin markers used to track the patient position, obtaining an intra-operative CBCT scan which is then automatically segmented with pedicles highlighted, planning desired screw trajectory in the axial, sagittal and coronal views, aligning and advancing the instrument along the planned path to the desired location in the vertebral body, obtaining intra-operative verification scan to check the final screw placement accuracy and need for any revision
Intraoperative CBCT and planned paths for screw placement were augmented to the video images showing the surgical field (Fig. 2). In case of patient movement, the planned paths were automatically corrected, without need for re-registration, owing to the continuous patient tracking by the optical skin markers. The surgeon placed the tip of the tracked Jamshidi needle (ClarifEye Needle, Philips) at the skin entry point and advanced it through the skin, and soft tissue until it reached the entry point on the vertebral body. As the optical marker on the shaft of the needle was at a pre-calibrated distance from the tip of the needle, tracking the on-shaft optical marker (Fig. 1) enabled depth tracking of the needle.
Based on the pathology being treated, screw placements and fusion procedures were either performed on their own or combined with decompression procedures, biopsy, cage placement and/or cement augmentation. When all screws were placed, intra-operative CBCT was performed to verify the screw positions. A screw was revised if the surgeon judged its position to be unsatisfactory. After revision, another CBCT was performed. At the end of the study, the final CBCT scans were collected, anonymized, and graded by three independent reviewers not involved in the procedure for accuracy according to the Gertzbein scale: grade 0 (screw within the pedicle without cortical breach), grade 1 (0–2 mm breach, minor perforation including cortical encroachment), grade 2 (2–4 mm breach, moderate breach, and grade 3 (> 4 mm breach, severe displacement) [12]. Neurophysiological monitoring was not performed during the procedure. Post-screw and rod placement, fascia and skin were closed in a standard fashion.
Post-operative CT was not performed because CBCT was considered sufficient for clinical evaluation [31].
The duration of different workflow steps and screw placement time were recorded. Staff dose was recorded using personalized dosimeters (DoseAware, Philips, Netherlands) and patient dose was recorded by the imaging system as Dose Area Product (DAP). The patient dose was calculated as the sum of the 2D and 3D component of the procedure and a post-instrumentation verification scan. Monte Carlo software (PCXMC v2.0, STUK, Helsinki, Finland) was used to estimate effective dose (ED) associated with given DAP [13].
SAS® Software Version 9.4 was used to calculate the sample size. Sample size calculation for the primary objective was based on a previous cadaver study [11] with 94% percutaneous pedicle screw placements accuracy in the thoracolumbarsacral spine. Assuming a similar accuracy as a previous cadaver study11 with an 8% margin of error and 85% power to detect a two-sided 95% confidence interval, using the Exact Clopper–Pearson test, we obtained a sample size of 200 screws. We further estimated a patient dropout rate of 10% which resulted in a final sample size of 220 screws.
The Gertzbein Grade of the placed screws was summarized using frequency counts and
percentages for the overall analysis set, by anatomical region (lumbar, sacral or thoracic), and by spinal level of the pedicles. For the overall analysis set, the proportion of accurately placed screws was summarized along with the 95% CI as calculated using Exact Clopper–Pearson (ignoring the clustering by subject or location of the pedicles) and Cochran method described by Zhou [14] accounting for the patient level clustering of the pedicles. Procedure time, staff and patient radiation dose were expressed as mean (± standard deviation), median (min–max range), or frequency (percentage), as appropriate.
Results
Forty patients were enrolled between July 2019 and March 2021. In total, 220 pedicle screws were placed. Thirty percent of the patients had previous spine surgeries and 15.4% were obese according to the WHO criteria. Of the 40 patients, one patient was excluded (four screws) as the navigation system could not be used because the sterile drape was stuck in the metal casing of the C-arm, blocking all C-arm movements. In one patient (with four screws), no verification scan was performed; therefore, the screws could not be rated for accuracy. Finally, one screw was not navigated because of local power outage. As a result, of the 220 screws, 211 screws were included in the final analysis of the study results. Of these, 32.7% were in the thoracic region 59.7% in the lumbar region, and 7.6% in the sacral region. Table 1 summarizes patient demographics and surgical indications. The distribution of screws at each vertebral level is shown in Fig. 3.
All surgeries were performed by two surgeons in Lugano and three surgeons in Germany. None of these surgeons had previous experience with the ON system, except during cadaver or phantom training sessions. However, all the surgeons had some experience with other navigation systems. Every patient received a minimum of 4 screws and a maximum of 8 screws (average of 5.5 screws per subject). Twenty-three patients had two-level fusion, four patients had three-level fusion, and 13 patients had four-level fusion.
No device related adverse events were observed during this study.
Accuracy
The screw placement accuracy (grade 0 and 1) was 98.1% (95%CI: 95.2%, 99.5%) using the exact method, corresponding to 207/211 successfully placed ((and 98.1% (95%CI:95.9%, 100%) using the cluster method. A total of 196, 11 and 4 screws were rated grades 0 (93%), 1 (5%), and 2 (2%), respectively. No screws were rated as severely misplaced (Grade 3). Figure 4 shows a breakdown of the screw accuracy for each Gertzbein grade for each anatomical region. All four misplaced screws had grade 2 medial breach. Two breaches were at T6, one at T7 and one at L4. All misplaced screws were in patients with unstable fractures.
Combined accuracy of screw placement for all regions and individual accuracies for thoracic, lumbar and sacral regions. In all regions of the spine, majority of the screws were perfectly placed (grade 0). There were no grade 3 screw placements. Majority (3) of the grade 2 screws were in the small pedicles in the thoracic region and 1 was observed in the lumbar spine
In the lumbar, thoracic, and sacral regions, 99.2, 95.7 and 100% of the screws, respectively, were accurately placed. Of the 211 screws placed, 6 were revised intraoperatively. None of the patients required postoperative screw revision prior to hospital discharge.
Screw navigation time
The average time from skin incision to skin closure was 2 h 50 min 28 s (± 1 h 9 min and 59 s) and included 27 cases in which the fusion was combined with discectomy, cage placement, laminectomy, biopsy and/or screw cementing. Because no invasive frame was used, the first skin incision was made at the start of the treatment step. Therefore, the skin incision to skin closure time, does not consider the time for preparation, acquisition, and planning steps, but includes the treatment duration which includes the time for all associated procedures, time for all verification scans, rod placement, and skin closure time. The average time out to skin closure time (including the preparation, acquisition, and planning in addition to time included as part of skin incision to skin closure) was 3 h 19 min 57 s (± 1 h 14 min and 41 s). The average time required to align the tracked Jamshidi needle, place K-wire, place the screw over the K-wire and tighten it in place was 6 min and 25 secs (± 3 min 33 secs). The time required for each individual workflow step and its relative proportion to the time out to skin closure time is provided in Tables 2, 3 and in Fig. 5.
Proportion of time out to skin closure time spent on each workflow step. The time out time is the surgical team's short pause when the patient is already positioned on the table, anesthetized, draped, and the surgery is about to begin. The team uses this moment to confirm that they are about to perform a specific surgery procedure on a specific anatomy and identifies the members of the staff involved in the procedure and present in the room. The pie chart represents the proportion of time spent in each workflow step of Augmented Reality Surgical Navigation as a percentage of the time out to skin closure time. Preparation step excludes time for draping the patient but includes time for isocentering patient and applying the skin markers. The treatment time duration includes the K-wire and screw placement time, as well as the time for decompression, cage placement, cementing, etc. when performed as a part of the procedure. The verification time includes the time for making the first verification scan as well as any additional optional verification scans that were done as a part of the intra-operative screw revision as well as the time required for optional repositioning of the screws. The “others” represent the time spent on rod placement, wound closure, etc.
An exploratory analysis divided patients in Lugano, where 31 of the 40 cases were performed, into three groups of consecutive 10 patients, based on their date of surgery. There was a tendency toward a shorter screw placement time (6 min 32 s to 3 min 59 s) when comparing the first and last 10 cases, suggesting that there may be reduced time required with increased physician experience with ON (Table 4).
Patient and staff radiation dose
The mean operator radiation dose per subject was 40.3 µSv. The mean patient effective dose (ED) was 11.94 mSv. The patient dose included the dose required for the planning and verification scans as well as the fluoroscopy dose. The overall mean relative contributions of fluoroscopy, planning, and verification scans to the total patient ED were 8.58%, 32.46% and 53.90%, respectively. Fluoroscopy was needed, despite the use of navigation, as in majority of cases, screw placement was combined with procedures such as cage placement, decompression, biopsy, and cement augmentation.
Discussion
This was the first clinical study in which ON was used for MISS pedicle screw placement. The obtained accuracy of 98.1% from 211 screws of which 32.7% were in thoracic region, in 39 patients with varying spine pathologies, 15.4% of those in the obese category and 30% had previous spine surgeries, with five different surgeons operating out of two separate hospitals in two different countries, strongly confirmed the safety and efficacy of this system.
Despite the continued rise in the adoption rates of MISS, very few clinical studies have specifically addressed the accuracy and clinical safety of such procedures with CAN, especially in the more challenging thoracic regions. Table 5 provides an overview of the accuracies of 3D navigation for MISS as reported in literature and the 98.1% accuracy obtained with the ON in this study, is in line with these published reports. The accuracy results of ON were also comparable to the pedicle screw placement accuracies of robotic systems [15,16,17].
In all the published reports, 3D navigation systems rely on bone-anchored reference frames to register the navigation system with the acquired 3D image data set [18]. Attaching the DRF can be challenging in obese patients and there is the possibility of inadvertent motion of the reference frame [18]. This may lead to registration errors and the need for re-registration, which may add to the procedure time. In addition, the inaccuracy increases with increasing distance from the reference frame [19]. ON relies on skin markers, which mitigate the issues described for bone-based reference frames. Global movements of the markers with respect to the underlying spine, such as during breathing, have no impact on accuracy. In cases where there is a local deformation due to stretching of the skin, ON focuses on tracking the remaining rigid part of the skin marker model to maintain tracking accuracy.
Skin-based tracking can be a cause for concern in obese patients who have larger amounts of adipose tissue and subsequently larger subcutaneous mobility [20]. In our study, we had 42 screws placed in 7 patients with a BMI > 30, which is the WHO cut-off for obesity, and 1 patient with a BMI > 60. All screws were placed accurately in these patients. The only other system relying on a non-invasive patient tracker in the form of a fixed form (unlike random skin marker placement with ON), an adhesive frame fitted with light emitting diodes (LED), which is applied to the patient’s back during spine surgery is the SpineMask (Stryker). However, to ensure the visibility of a sufficient number of LEDs for tracking via infra-red cameras, this noninvasive patient tracker mask requires a larger intraoperative 3D image dataset which has been shown to result in a higher radiation exposure to the patient [21]
One of the issues associated with the use of navigation systems is the time required to set it up. The mean setting up time for ON (which included time for placing skin markers, obtaining 3D scan and segmenting the vertebrae to create a 3D model) was 15 min 26 s. This was lower than the 30 min preparation time reported by Shin et al. [22] and 33.6 min recorded by Ille et al. [23] for the O-arm. The reduced preparation time could be explained by following factors: (i) As a skin marker-based system, no additional time is needed to attach the DRF (ii) combining imaging and navigation into one system eliminates time needed to register and transfer the data between systems. In addition, no extra time is spent in re-registration (iii) tracked needle was pre-calibrated and so no time was needed for registering and calibrating the instrument (iv) there was no additional time required to position the cameras to ensure a clear line of sight as the cameras were integrated in the C-arm detector.
The longest part of the procedure time was devoted to the treatment time which included the time required to navigate the pedicle screw in the optimum position as well as the time required to carry out non-fusion related steps such as decompression, biopsy, cement augmentation and placement of rods. On average, the treatment time contributed 45% of the total procedure time. The mean time required to place screws in optimum position was lower than or comparable to values reported in the literature for open and MIS lumbosacral pedicle screw placements [5, 23, 25]. However, there was a large variation in the screw placement times which could be a consequence of the learning curve effect.
The hazards of increased radiation exposure to staff are well known [23]. The average operator radiation dose was 40.3 µSv in the present study. In a study where 2D fluoroscopy was used to place pedicle screws in 24 patients via a minimally invasive TLIF procedure, surgeons received several times higher radiation doses averaging 270 µSv, which is significantly higher than what was observed in our study and even underestimates the exposure to the staff, since the dosimeter was placed under the lead apron at the waist level [26].
Patient radiation dose is another aspect of concern when using CAN. The reported effective average radiation dose to the patient when using the Airo CT (Stryker) is 5.5 to 7.4 mSv per scan based on patient weight [27]. Corresponding average radiation dose for O-arm (Medtronic) is reported to be between 8 and 9 mSv per scan when using the High Dose scan [28, 29]. In the current study, the average ED for making planning scans was 3.65 mSv and the mean ED for the whole procedure was 11.94 mSv, based on an average of 2.4 scans per patient as well as the dose for fluoroscopy used during the remaining procedure (cage placement, cementing, etc.). Other studies have reported doses up to 31 mSv when maximum of three scans are performed during a procedure.30 While indicative of the lower radiation dose of ON, an accurate comparison between different systems will require a study with the available CAN systems on phantoms or cadavers of similar BMI.
Moreover, publications indicate that the image quality of the intra-operative CBCT with the ON is as reliable as the image quality of a conventional CT scan for pedicle screw assessments. 31 Therefore, the post-op CT scan was replaced with an intra-operative verification CBCT which resulted in dose savings 31 and potentially avoiding repeat surgeries to reposition misplaced screws.
The main limitation of this study is that it does not compare ON to fluoroscopy or other navigation technologies. Also, due to limited cohort size, no definitive conclusion on learning curve associated with ON could be made.
Conclusion
ON with non-invasive skin markers can be used to achieve high accuracy (98.1%) for screw placement during MISS in thoracolumbarsacral with acceptable screw placement times and patient radiation.
Change history
20 October 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00586-023-07989-7
References
Schizas C, Michel J, Kosmopoulos V, Theumann N (2007) Computer tomography assessment of pedicle screw insertion in percutaneous posterior transpedicular stabilization. Eur Spine J 16:613–617
Bourgeois AC et al. (2015) Improved accuracy of minimally invasive transpedicular screw placement in the lumbar spine with 3-dimensional stereotactic image guidance: a comparative meta-analysis. J Spinal Disord Tech 28:324–329
Innocenzi G, Bistazzoni S, D’Ercole M, Cardarelli G, Ricciardi F (2017) Does navigation improve pedicle screw placement accuracy? Comparison between navigated and non-navigated percutaneous and open fixations. Acta Neurochir Suppl 124:289–295
Ohba T, Ebata S, Fujita K, Sato H, Haro H (2016) Percutaneous pedicle screw placements: accuracy and rates of cranial facet joint violation using conventional fluoroscopy compared with intraoperative three-dimensional computed tomography computer navigation. Eur Spine J 25(6):1775–1780. https://doi.org/10.1007/s00586-016-4489-1
Ding BTK, Kaliya-Perumal AK, Oh JY, Yu CS (2020) Prospective evaluation of the time required for insertion of 380 lumbar and sacral pedicle screws using navigation with an intraoperative 3- dimensional imaging system. Int J Spine Surg 14(3):368–374. Published 2020 Jun 30. Doi: https://doi.org/10.14444/7048
Nakashima H, Sato K, Ando T, Inoh H, Nakamura H (2009) Comparison of the percutaneous screw placement precision of isocentric C-arm 3-dimensional fluoroscopy-navigated pedicle screw implantation and conventional fluoroscopy method with minimally invasive surgery. J Spinal Disord Tech 22(7):468–472. https://doi.org/10.1097/BSD.0b013e31819877c8
Yang BP, Wahl MM, Idler CS (2012) Percutaneous lumbar pedicle screw placement aided by computer-assisted fluoroscopy-based navigation: perioperative results of a prospective, comparative, multicenter study. Spine (Phila Pa 1976) 37(24):2055–2060. Doi: https://doi.org/10.1097/BRS.0b013e31825c05cd
Overley SC, Cho SK, Mehta AI et al (2017) Navigation and robotics in spinal surgery: Where are we now? Neurosurgery 80:S86-99
Tian NF, Huang QS, Zhou P et al (2011) Pedicle screw insertion accuracy with different assisted methods: a systematic review and meta-analysis of comparative studies. Eur Spine J 20(6):846–859. https://doi.org/10.1007/s00586-010-1577-5
Elmi-Terander A, Burström G, Nachabe R, Skulason H, Pedersen K, Fagerlund M, Ståhl F, Charalampidis A, Söderman M, Holmin S, Babic D, Jenniskens I, Edström E, Gerdhem P (2019) Pedicle screw placement using augmented reality surgical navigation with intraoperative 3d imaging: a first in-human prospective cohort study. Spine (Phila Pa 1976). 44(7):517–525. Doi: https://doi.org/10.1097/BRS.0000000000002876. PMID: 30234816; PMCID: PMC6426349
Peh S, Chatterjea A, Pfarr J, Schäfer JP, Weuster M, Klüter T, Seekamp A, Lippross S (2020) Accuracy of augmented reality surgical navigation for minimally invasive pedicle screw insertion in the thoracic and lumbar spine with a new tracking device. Spine J 20(4):629–637. https://doi.org/10.1016/j.spinee.2019.12.009 (Epub 2019 Dec 19 PMID: 31863933)
Gertzbein SD, Robbins SE (1990) Accuracy of pedicular screw placement in vivo. Spine (Phila Pa 1976) 15:11–14
Scarone P, Vincenzo G, Distefano D, Del Grande F, Cianfoni A, Presilla S, Reinert M (2018) Use of the Airo mobile intraoperative CT system versus the O-arm for transpedicular screw fixation in the thoracic and lumbar spine: a retrospective cohort study of 263 patients. J Neurosurg Spine 29(4):397–406. https://doi.org/10.3171/2018.1.SPINE17927 (Epub 2018 Jul 6 PMID: 29979141)
Zhou X, Obuchowski NA, McClish DK (2011). Statistical methods in diagnostic medicine, 2nd edition, pp 110–111
Tsai TH, Wu DS, Su YF, Wu CH, Lin CL (2016) A retrospective study to validate an intraoperative robotic classification system for assessing the accuracy of kirschner wire (K-wire) placements with postoperative computed tomography classification system for assessing the accuracy of pedicle screw placements. Medicine (Baltimore) 95:e4834. https://doi.org/10.1097/MD.0000000000004834
Keric N, Doenitz C, Haj A, Rachwal-Czyzewicz I, Renovanz M, Wesp DMA et al (2017) Evaluation of robot-guided minimally invasive implantation of 2067 pedicle screws. Neurosurg Focus 42:E11. https://doi.org/10.3171/2017.2.FOCUS16552
Shafi KA, Pompeu YA, Vaishnav AS, Mai E, Sivaganesan A, Shahi P, Qureshi SA (2022) Does robot-assisted navigation influence pedicle screw selection and accuracy in minimally invasive spine surgery? Neurosurg Focus 52(1):E4
Malham GM, Wells-Quinn T (2019) What should my hospital buy next?-Guidelines for the acquisition and application of imaging, navigation, and robotics for spine surgery. J Spine Surg 5(1):155–165. Doi: https://doi.org/10.21037/jss.2019.02.04. PMID: 31032450; PMCID: PMC6465454
Cho JY, Chan CK, Lee SH, Lee HY (2012) The accuracy of 3D image navigation with a cutaneously fixed dynamic reference frame in minimally invasive transforaminal lumbar interbody fusion. Comput Aided Surg 17(6):300–309
Seidell JC, Flegal KM (1997) Assessing obesity: classification and epidemiology. Br Med Bull 53(2):238–252. https://doi.org/10.1093/oxfordjournals.bmb.a011611 (PMID: 9246834)
Klingler JH, Hubbe U, Scholz C, Volz F, Hohenhaus M, Vasilikos I, Masalha W, Watzlawick R, Naseri Y (2020) Noninvasive patient tracker mask for spinal 3D navigation: does the required large-volume 3D scan involve a considerably increased radiation exposure? J Neurosurg Spine 28:1–7. Doi: https://doi.org/10.3171/2020.5.SPINE20530. Epub ahead of print. PMID: 32858517.
Shin MH, Ryu KS, Park CK Accuracy and safety in pedicle screw placement in the thoracic and lumbar spines : comparison study between conventional C-arm fluoroscopy and navigation coupled with O-arm® guided method
Siasios ID, Pollina J, Khan A, Dimopoulos VG (2017) Percutaneous screw placement in the lumbar spine with a modified guidance technique based on 3D CT navigation system. J Spine Surg 3(4):657–665. https://doi.org/10.21037/jss.2017.12.05
Jenkins NW, Parrish JM, Sheha ED, Singh K (2021) Intraoperative risks of radiation exposure for the surgeon and patient. Ann Transl Med 9(1):84. https://doi.org/10.21037/atm-20-1052
Spitz SM, Sandhu FA, Voyadzis JM (2015) Percutaneous “K-wireless” pedicle screw fixation technique: an evaluation of the initial experience of 100 screws with assessment of accuracy, radiation exposure, and procedure time. J Neurosurg Spine 22:422–431. https://doi.org/10.3171/2014.11.SPINE14181
Hubbe U, Sircar R, Scheiwe C, Scholz C, Kogias E, Krüger MT, Volz F, Klingler JH (2015) Surgeon, staff, and patient radiation exposure in minimally invasive transforaminal lumbar interbody fusion: impact of 3D fluoroscopy-based navigation partially replacing conventional fluoroscopy: study protocol for a randomized controlled trial. Trials 9(16):142. https://doi.org/10.1186/s13063-015-0690-5
Navarro-Ramirez R, Lang G, Lian X et al (2017) Total navigation in spine surgery; a concise guide to eliminate fluoroscopy using a portable intraoperative computed tomography 3-dimensional navigation system. World Neurosurg 100:325–335
Kim TT, Drazin D, Shweikeh F, Pashman R, Johnson JP (2014) Clinical and radiographic outcomes of minimally invasive percutaneous pedicle screw placement with intraoperative CT (O-arm) image guidance navigation. Neurosurg Focus 36:E1
Oertel MF, Hobart J, Stein M, Schreiber V, Scharbrodt W (2011) Clinical and methodological precision of spinal navigation assisted by 3D intraoperative O-arm radiographic imaging. J Neurosurg Spine 14:532–536
Lange J, Karellas A, Street J et al. Estimating the effective radiation dose imparted to patients by intraoperative cone-beam computed tomography in thoracolumbar spinal surgery
Burström G, Cewe P, Charalampidis A, Nachabe R, Söderman M, Gerdhem P, Elmi-Terander A, Edström E (2021) Intraoperative cone beam computed tomography is as reliable as conventional computed tomography for identification of pedicle screw breach in thoracolumbar spine surgery. Eur Radiol 31(4):2349–2356. https://doi.org/10.1007/s00330-020-07315-5
Acknowledgements
Philips Healthcare, the Netherlands funds were received in support of this work.
Funding
Philips Healthcare Netherlands funds were received in support of this work. Dr Pietro Scarone and Prof Andreas Seekamp have consultant agreements with Philips. Anindita Chatterjea and Inge Jenniskens are employed by Philips. The devices used in the study are FDA-approved or approved by corresponding national agency for this indication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they do not have relationships/conditions/circumstances that present potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Scarone, P., Chatterjea, A., Jenniskens, I. et al. Percutaneous thoraco-lumbar-sacral pedicle screw placement accuracy results from a multi-center, prospective clinical study using a skin marker-based optical navigation system. Eur Spine J 31, 3098–3108 (2022). https://doi.org/10.1007/s00586-022-07387-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-022-07387-5