Abstract
Purpose
Degenerative cervical myelopathy (DCM) is the most common non-traumatic cause of spinal cord dysfunction. Prediction of the neurological outcome after surgery is important. The aim of this study was to analyze the relationship between first symptoms of DCM and the neurological outcome after surgery.
Methods
A retrospective analysis over a period of 10 years was performed. First symptoms such as cervicobrachial neuralgia, sensory and motor deficits and gait disturbances were evaluated regarding the postoperative neurological outcome. The modified Japanese Orthopedic Association Score (mJOA Score) was used to evaluate neurological outcome.
Results
In total, 411 patients (263 males, 64%) with a median age of 62.6 ± 12.1 years were included. Cervicobrachial neuralgia was described in 40.2%, gait disturbance in 31.6%, sensory deficits in 19% and motor deficits in 9.2% as first symptom. Patients with cervicobrachial neuralgia were significantly younger (median age of 58 years, p = 0.0005) than patients with gait disturbances (median age of 68 years, p = 0.0005). Patients with gait disturbances and motor deficits as first symptom showed significantly lower mJOA Scores than other patients (p = 0.0005). Additionally, motor deficits and gait disturbance were negative predictors for postoperative outcome according to the mJOA Score.
Conclusion
Motor deficits and gait disturbances as the first symptom of DCM are negative predictors for postoperative neurological outcome. Nevertheless, patients with motor deficits and gait disturbance significantly profit from the surgical treatment despite poor preoperative mJOA Score.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Degenerative cervical myelopathy (DCM) is an age-dependent deterioration of the spinal cord with an increasing epidemiologic relevance. It is the most common non-traumatic cause of spinal cord dysfunction in adults [1, 2], with a great impact on worldwide health, society and economy [3]. Approximately 1.6 per 100,000 inhabitants per annum require surgical treatment for symptomatic DCM [4].
The variable presentation of the DCM is an expression of the complex interaction of mechanic and vascular factors. The degenerative process with a progressive spinal stenosis leads to an ongoing static compression of the spinal cord and the nerve roots with subsequent demyelination. This may result in necrosis of both, gray and white matter. In addition, a dynamic component resulting through the mobility of the cervical spine might increase the pressure on the spinal cord [5, 6].
Moreover, vascular factors are discussed to cause myelopathy due to a reduced blood supply or due to a reduced venal drain caused by a higher pressure due to the chronic and progressive compression of the spinal cord. Furthermore, a chronic pressure induces the activation of microglia and the recruitment of macrophages at the site of the compression and leads to a neuroinflammation [7]. Additionally, genetic predisposition causing the degenerative spondylotic transformation has been discussed in the current literature [8].
Surgical treatment is recommended for severe and moderate DCM, while the treatment of mild DCM is still discussed [9]. In particular, Kalsi-Ryan et al. showed that patients with mild DCM failed to improve their neurological function significantly after structured conservative treatment and that 23–54% of those patients underwent delayed surgical treatment [10].
The clinical presentation of DCM is various and in general unspecific at the early beginning. Therefore, the clinical diagnosis of mild DCM might be difficult and delayed due to comorbidities mimicking DCM. The patients usually suffer from neck and shoulder pain with or without radiculopathy, numbness and fine motor deficits, ataxic gait disturbance, and sphincter dysfunction [11]. Additionally, hyperreflexia, a positive Hoffmann’s sign, and general weakness may be signs of myelopathy [12, 13]. Furthermore, DCM and its neurological symptoms can be stable for a long period of time and deteriorate episodically [2, 14].
Several predictors like duration of symptoms, high signal intensity (SI) on T2-weighted MRI, preoperative modified Japanese Orthopedic Association Score (mJOA Score), age, comorbidities, or smoking status were identified over the recent years to influence the neurological outcome of DCM patients. Interestingly, other factors like the type of stenosis, the number of affected levels, ventral or posterior approach or the type of surgical treatment do not affect the postoperative neurological outcome [13,14,15,16,17,18]. To our best knowledge, first symptom of DCM was not evaluated as a possible predictor of neurological outcome after surgery in detail. Therefore, we aimed to analyze the first symptoms of patients suffering from DCM and to evaluate possible correlation between the first symptom and the neurological outcome after surgery.
Patients and methods
Patients and clinical data
Clinical data of patients suffering from cervical degenerative disorders treated surgically in our department between 2007 and 2016 were retrospectively analyzed. All patients with DCM were included. Patients with other diseases, which might induce a myelopathy, were excluded: congenital abnormalities of the cervical spine, metastatic diseases, rheumatoid disorders, fractures of cervical vertebral bodies, traumatic spinal cord injury or myelopathy in relation with a cervical spine instability.
The study was conducted in accordance with the STROBE guidelines after the approval by the Institutional Review Board (Medical Faculty, University of Duisburg-Essen, Registration number: 16–6270-BO).
Neurological symptoms
Neurological symptoms were recorded at admission and divided into cervicobrachial neuralgia, sensory deficits, motor deficits and gait disturbance. The preoperative status and the postoperative outcome were analyzed during in-patient treatment, and at three and six months after surgery. The mJOA Score [19] was used for neurological assessment.
Statistics
The data were analyzed using SPSS 25.0 (Statistical Package for the Social Sciences, SPSS Inc., Chicago, IL). Metrics were described by median and range and nominal data by frequency and valid percentage. P values < 0.05 in two-sided testing were considered significant.
Demographics, clinical, and radiographic parameters were analyzed in a univariate way regarding their association or correlation with pre- and postoperative mJOA Scores. Pearson Chi2 statistics or Fischer exact test was used for dichotomous variables. As the data were not normally distributed, Kendall-Tau-b was assessed for continuous and ordinal variables, Spearman Rho for continuous and dichotomous, and Mann–Whitney U test for ordinal and continuous variables. Significant parameters selected through univariate analysis as well as parameters with P values < 0.1 were subsequently evaluated using multivariate analysis.
A multiple regression analysis was then conducted, wherein mJOA Score was considered a continuous variable. A stringent confidence level of 99% was used. Therefore, only P values < 0.01 were considered significant. Patients, who were lost to follow-up, were not included in statistical analyses at those time points.
Results
Demographics
We included 411 patients with a median age of 62.6 ± 12.1 years (range from 31 to 96 years). Of those, 263 patients were males (64%), and 148 patients were females (36%). DCM developed due to a spinal stenosis in 249 patients (60.6%), and due to a herniated disk in 162 patients (39.4%). A high SI on T2-weighted MRI was detected in 248 patients (60.3%).
First neurological symptom
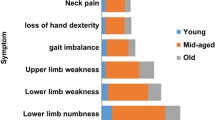
Cervicobrachial neuralgia was the most common first symptom in majority of the patients suffering from DCM (n = 165, 40.2%). Gait disturbance was described less frequently (n = 130, 31.6%). Sensory deficits and motor deficits were seen in 78 patients (19%) and in 38 patients (9.2%) as the first symptom of DCM (Fig. 1).
First neurological symptom and symptom duration
Symptom duration until surgery in patients with motor deficits was significantly shorter than in patients without motor deficits as the first symptom (7.5 weeks versus 16 weeks, p = 0.002) Interestingly, diagnosis of DCM in patients with gait disturbances took the longest time with an average of 19.5 weeks, showing a significant difference between patients without gait disturbance as the first symptom of DCM (16 weeks, p = 0.046). Patients with cervicobrachial neuralgia and sensory deficits as descripted first symptom admitted after 15–16 weeks in average (Table 1).
First neurological symptom and demographic characteristics
Patients with cervicobrachial neuralgia were significantly younger (median age: 58 years) than patients without cervicobrachial neuralgia as the first symptoms (median age: 66 years, p = 0.0005). Significant difference in age was also seen in patients with gait disturbance (median age: 68 years) versus patients without gait disturbance (median age: 60 years, p = 0.0005). Sensory deficits and motor deficits showed no significant differences in age. Regarding the patients’ sex, the statistical analysis showed no significant association with the first symptom of DCM (Table 1).
First neurological symptom and high SI on T2-weighted MRI
High SI on T2-weighted MRI was most common in patients with motor deficits (n = 28/38, 73.7%), but without significant correlation (p = 0.084). A high SI on T2-weighted MRI was present in majority of patients with cervicobrachial neuralgia (n = 96/165 patients, 58.2%), gait disturbances (n = 79/130, 60.8%) and sensory deficits (n = 45/78, 57.7%, Table 1).
First neurological symptom in relation with median mJOA Score
Median mJOA Score was significant higher in patients with cervicobrachial neuralgia as the first symptom than in patients with other symptoms (median 15 versus 14 preoperative and median 18 versus 17 6 months postoperative, p = 0.0005) at all times of observation. The median mJOA Score did not differ significantly in patients with or without sensory deficits as first symptom, whereas patients with motor deficits as first symptom showed significant lower preoperative mJOA Scores than patients without motor deficits as first symptom (median 12.5 versus 15, p = 0.0005). During the postoperative follow-up, there was an improvement of the mJOA Score in that cohort (median: 12.5–16.5), but the significant difference compared to patients without motor deficits remained (16.5 versus 17, p = 0.0005). Although the patients with gait disturbance as first symptom showed significantly lower initial mJOA Scores (median 14 versus 15, p = 0.0005), the postoperative improvement was comparable in all subgroups (mJOA score improvement by 3 points) regarding the first clinical symptom (Table 2).
Multivariate analysis
Multiple regression analysis of the first symptoms of DCM showed that motor deficits are negative predictors for worse neurological outcome at all times of observation according to the mJOA Score. Gait disturbance was also a negative predictor for worse neurological outcome preoperative, postoperative and 3 months after surgery. Cervicobrachial neuralgia was not associated with a worse neurological outcome (Table 3).
Lost to follow-up
The lost to follow-up was 6.6% (27 patients) three months after surgery and 23.1% (95 patients) six months after surgery.
Discussion
The best treatment of mild DCM is still a matter of debate [9]. Fehlings et al. identified regional differences in demographics, severity of myelopathy and extent of postoperative improvements [20], which makes recommendations of therapy even more difficult. It is possible that the severity of symptoms does not change over a long time, but episodic deterioration is described [2, 14]. Therefore, the timeframe of surgical intervention varies. Due to the variety of different symptoms and its expression, timely diagnosis of DCM, especially of mild DCM, might be difficult in some cases [18, 21, 22]. The most common symptoms of DCM are cervicobrachial neuralgia, sensory deficits, motor deficits and gait disturbance [1].
Age, preoperative mJOA Score and the high SI on T2-weighted MRI are known to have a negative influence on the postoperative neurological outcome [16,17,18]. Evaluation of the first symptoms and their influence on the neurological outcome could also play an important role in decision making, especially in timing of surgery.
Symptom duration was described as a negative outcome predictor in several studies [18, 23, 24]. Holly et al. highlight the importance of symptom duration and age for the postoperative outcome of DCM patients [17]. In contrast, Zika et al. found no correlation between symptom duration and neurological outcome after surgery for DCM [25].
The onset of the first symptoms of DCM might be unspecific and covered by comorbidities. In our cohort, patients with severe motor deficits showed a significant shorter period from the beginning of symptoms until surgery of DCM (7.5 weeks) than patients without motor deficits as first symptom (16 weeks). Contrary, DCM was detected later in patients with unspecific cervicobrachial neuralgia or gait disturbance. Gait disturbances had the longest period with 19.5 weeks. The age-dependent physical weakness in majority of the elderly patients and the known comorbidities such as hip and knee osteoarthritis, cerebral vascular disorders, diabetic neuropathy, benign prostatic hypertrophy, or urinary stress incontinence, or known entrapment of peripheral neuropathy (carpal or cubital tunnel syndrome) might mimic symptoms of DCM and therefore, prolong its diagnosis [26].
In our analysis, symptom duration was not a predictor for worse neurological outcome. The favorable recovery of preoperative deficits and the wide variance of symptom duration until surgery (1 until 350 weeks, median duration: 35.4 weeks) could be reasonable. Additionally, the retrospective character of the study itself could bias the results and explain the different results compared to prospective analysis of the current literature.
Cervicobrachial neuralgia was the most common first symptom in our cohort. The preoperative and postoperative mJOA Score was significantly higher in those patients than in patients with other first symptoms (Table 2). Cervicobrachial neuralgia was seen in majority of mild DCM (Table 1). Therefore, cervicobrachial neuralgia failed to be a predictor for a worse neurological outcome. Nevertheless, Kadanka Jr. et al. were able to show in a prospective observational follow-up study of 112 patients suffering from “non-myelopathic” degenerative cervical cord compression that radiculopathy is an independent significant predictor for progression into symptomatic DCM [27]. Therefore, ongoing cervicobrachial neuralgia in mild DCM should be consequently followed-up if conservative therapy is performed. Surgery might be offered in those cases to prevent possible deterioration of neurological symptoms.
Preoperative mJOA Score is known to be a negative predictor for postoperative neurological outcome [17, 18, 28]. Those results are comparable with our study. Baseline mJOA Score was significant less in patients with motor deficits compared to patients without motor deficits (12.5 versus 15, p = 0.0005), and in patients with gait disturbance compared to patients without gait disturbance (14 versus 15, p = 0.0005). Therefore, preoperative motor deficit as the first symptom of DCM was an independent predictor for worse neurological outcome over the complete observational period, while gait disturbance was an independent predictor of worse postoperative neurological outcome until three months postoperative. However, the postoperative mJOA Score in patients with motor deficits as first symptom of DCM improved comparable to the patients with cervicobrachial neuralgia or sensory deficits as the first symptom. Surgical treatment and the significant shorter symptom duration until surgery (7.5 weeks for patients with motor deficits versus 16 weeks for the other patients) might have played a role in the favorable postoperative, but still worse recovery of patients with preoperative motor deficits and gait disturbance. Additionally, cervicobrachial neuralgia and sensory deficits do not predict the neurological outcome, because they do not influence the mJOA Score in a way motor deficits or gait disturbance do.
Neurological recovery in our study is in accordance with the results of Goh et al., who were able to show that patients with a severe DCM showed significantly greater improvement in JOA, Neurogenic Symptoms, Neck Disability Index, SF-36 Physical Component Summary, and Mental Component Summary and a larger proportion attained Minimal clinically important difference for Neck Disability Index and SF-36 Physical Component Summary after surgery than patients with moderate and mild DCM, while Minimal clinically important difference was equal for JOA Score [29]. Furthermore, surgical intervention for DCM is reducing the incidence rate of falls (decreased significantly from 497.4 to 90.3 falls per 100 person-years) and the incidence of motor deterioration per fall (decreased significantly from 34 to 8%) [30]. Surgery can improve motor deficits and decrease nursing care requirements among elderly patients with DCM [31]. Therefore, early surgery might be the treatment of choice to interfere the natural history of DCM and improve the neurological prognosis [15, 16, 18, 21].
The signal change in T1- and T2-weighted MRI is well known in DCM [12, 32, 33], but might be absent in 21% of patients [34]. The high SI on T2-weighted MRI has been attributed to edema, inflammation, gliosis and myelomalacia after long-standing compression of the spinal cord [35]. There is a significant correlation between T2-weighted SI and the degree of spinal cord compression [12, 32]. Furthermore, high SI on T2-weighted MRI is known to be a negative predictor for neurological outcome [16, 36, 37]. For example, Vedantam et al. demonstrated that a high SI in T2- weighted MRI is associated with a lower rate on postoperative recovery [38]. Gibson et al. reported a high correlation of Babinski sign, Hoffman’s sign, inverted brachioradialis reflex and hyperreflexia with the myelopathy and the consecutive spinal cord damage [13]. However, in our cohort the symptoms at the onset of DCM showed no significant correlation with SI in T2- weighted MRI, but a statistical trend was seen in patients with motor deficits. The statistical trend that motor deficits were associated with a significant higher amount of signal changes on T2-weighted MRI might be caused by the small number of patients presented with motor deficits as first symptom of DCM. Only 38 patients (9.2%) suffered from motor deficits as first symptom of DCM. Of those, 73.7% showed high SI on T2-weighted MRI compared to patients with cervicobrachial neuralgia (58.2%), gait disturbances (60.8%) and sensory deficits (57.7%). Therefore, significance might be reached in a cohort with more patients suffering from motor deficits. Additionally, cervicobrachial neuralgia, sensory deficits and gait disturbance are symptoms, which might not result from SI changes on MRI as a result of spinal cord damage alone. Those symptoms are influenced by multiple other causes such as comorbidities like diabetes mellitus or polyneuropathy, age, and simply mislead of the cervical spine.
Study limitations
The main limitation of our study is the retrospective, non-randomized character of the study with its associated inherent bias. The incomplete follow-up and the short follow-up period of six months limited the prediction and carries the risk of additional information and selection bias. However, future prospective studies with a longer follow-up are needed to evaluate the neurological long-term outcome and to strengthen the predictive analysis in patients.
Conclusion
Patients with motor deficits and gait disturbance as first symptom of DCM showed significant lower mJOA Scores than patients with cervicobrachial neuralgia or sensory deficits and are, therefore, negative predictors for postoperative neurological outcome. Nevertheless, patients with motor deficits and gait disturbance might significantly profit from the surgical treatment, despite lower preoperative mJOA Score.
Abbreviations
- DCM:
-
Degenerative cervical myelopathy
- mJOA:
-
Modified Japanese Orthopedic Association Score
- SI:
-
Signal intensity
References
Zileli M, Borkar SA, Sinha S et al (2019) Cervical spondylotic myelopathy: natural course and the value of diagnostic techniques -wfns spine committee recommendations. Neurospine 16(3):386–402
Baron EM, Young WF (2007) Cervical spondylotic myelopathy: a brief review of its pathophysiology, clinical course, and diagnosis. Neurosurgery 60(Suppl 2):S35-41
Kleinman N, Patel AA, Benson C et al (2014) Economic burden of back and neck pain: effect of a neuropathic component. Popul Health Manag 17(4):224–232
Boogaarts HD, Bartels RH (2015) Prevalence of cervical spondylotic myelopathy. Eur Spine J 24(Suppl 2):139–141
Inukai T, Uchida K, Nakajima H et al (2009) Tumor necrosis factor-alpha and its receptors contribute to apoptosis of oligodendrocytes in the spinal cord of spinal hyperostotic mouse (twy/twy) sustaining chronic mechanical compression. Spine (Phila Pa 1976) 34(26):2848–2857
Matz PG, Anderson PA, Holly LT et al (2009) The natural history of cervical spondylotic myelopathy. J Neurosurg Spine 11(2):104–111
Karadimas SK, Moon ES, Yu WR et al (2013) A novel experimental model of cervical spondylotic myelopathy (CSM) to facilitate translational research. Neurobiol Dis 54:43–58
Nouri A, Tetreault L, Singh A et al (2015) Degenerative cervical myelopathy: epidemiology, genetics, and pathogenesis. Spine (Phila Pa 1976) 40(12):E675-693
Fehlings MG, Tetreault LA, Riew KD et al (2017) A clinical practice guideline for the management of patients with degenerative cervical myelopathy: recommendations for patients with mild, moderate, and severe disease and nonmyelopathic patients with evidence of cord compression. Global Spine J 7(3 Suppl):70S-83S
Kalsi-Ryan S, Clout J, Rostami P et al (2019) Duration of symptoms in the quantification of upper limb disability and impairment for individuals with mild degenerative cervical myelopathy (DCM). PLoS One 14(9):e0222134
Badhiwala JH, Ahuja CS, Akbar MA et al (2020) Degenerative cervical myelopathy - update and future directions. Nat Rev Neurol 16(2):108–124
Nouri A, Tetreault L, Dalzell K et al (2017) the relationship between preoperative clinical presentation and quantitative magnetic resonance imaging features in patients with degenerative cervical myelopathy. Neurosurgery 80(1):121–128
Gibson J, Nouri A, Krueger B et al (2018) Degenerative cervical myelopathy: a clinical review. Yale J Biol Med 91(1):43–48
Lees F, Turner JW (1963) Natural History and prognosis of cervical spondylosis. Br Med J 2(5373):1607–1610
Gembruch O, Jabbarli R, Rashidi A et al (2019) Degenerative cervical myelopathy in higher-aged patients: How do they benefit from surgery? J Clin Med 9(1):62
Gembruch O, Jabbarli R, Rashidi A et al (2020) Surgery for degenerative cervical myelopathy: What really counts? Spine (Phila Pa 1976) 46(5):294–299
Holly LT, Matz PG, Anderson PA et al (2009) Clinical prognostic indicators of surgical outcome in cervical spondylotic myelopathy. J Neurosurg Spine 11(2):112–118
Tetreault LA, Karpova A, Fehlings MG (2015) Predictors of outcome in patients with degenerative cervical spondylotic myelopathy undergoing surgical treatment: results of a systematic review. Eur Spine J 24(Suppl 2):236–251
Benzel EC, Lancon J, Kesterson L, Hadden T (1991) Cervical laminectomy and dentate ligament section for cervical spondylotic myelopathy. J Spinal Disord 4(3):286–295
Fehlings MG, Kopjar B, Ibrahim A et al (2018) Geographic variations in clinical presentation and outcomes of decompressive surgery in patients with symptomatic degenerative cervical myelopathy: analysis of a prospective, international multicenter cohort study of 757 patients. Spine J 18(4):593–605
Fehlings MG, Ibrahim A, Tetreault L et al (2015) A global perspective on the outcomes of surgical decompression in patients with cervical spondylotic myelopathy: results from the prospective multicenter AOSpine international study on 479 patients. Spine (Phila Pa 1976) 40(17):1322–1328
Mattei TA, Goulart CR, Milano JB et al (2011) Cervical spondylotic myelopathy: pathophysiology, diagnosis, and surgical techniques. ISRN Neurol 2011:463729
Tetreault L, Wilson JR, Kotter MRN et al (2019) Is preoperative duration of symptoms a significant predictor of functional outcomes in patients undergoing surgery for the treatment of degenerative cervical myelopathy? Neurosurgery 85(5):642–647
Li XY, Lu SB, Sun XY et al (2018) Clinical and magnetic resonance imaging predictors of the surgical outcomes of patients with cervical spondylotic myelopathy. Clin Neurol Neurosurg 174:137–143
Zika J, Alexiou GA, Giannopoulos S et al (2020) Outcome factors in surgically treated patients for cervical spondylotic myelopathy. J Spinal Cord Med 43(2):206–210
Tanaka J, Seki N, Tokimura F, Doi K, Inoue S (1999) Operative results of canal-expansive laminoplasty for cervical spondylotic myelopathy in elderly patients. Spine (Phila Pa 1976) 24(22):2308–2312
Kadanka Z Jr, Adamova B, Kerkovsky M et al (2017) Predictors of symptomatic myelopathy in degenerative cervical spinal cord compression. Brain Behav 7(9):e00797
Madhavan K, Chieng LO, Foong H, Wang MY (2016) Surgical outcomes of elderly patients with cervical spondylotic myelopathy: a meta-analysis of studies reporting on 2868 patients. Neurosurg Focus 40(6):E13
Goh GS, Liow MHL, Ling ZM et al (2020) Severity of preoperative myelopathy symptoms affects patient-reported outcomes, satisfaction, and return to work after anterior cervical discectomy and fusion for degenerative cervical myelopathy. Spine (Phila Pa 1976) 45(10):649–656
Kimura A, Takeshita K, Shiraishi Y et al (2020) Effectiveness of surgical treatment for degenerative cervical myelopathy in preventing falls and fall-related neurological deterioration: a prospective multi-institutional study. Spine (Phila Pa 1976) 45(11):E631–E638
Yoshida G, Kanemura T, Ishikawa Y et al (2013) The effects of surgery on locomotion in elderly patients with cervical spondylotic myelopathy. Eur Spine J 22(11):2545–2551
Yu L, Zhang Z, Ding Q et al (2015) Relationship between signal changes on T2-weighted magnetic resonance images and cervical dynamics in cervical spondylotic myelopathy. J Spinal Disord Tech 28(6):E365-367
De la Garza RR, Nouri A, Nakhla J et al (2019) Predictors of return to normal neurological function after surgery for moderate and severe degenerative cervical myelopathy: an analysis of a global AOSpine cohort of patients. Neurosurgery 85(5):E917–E923
Rhee JM, Heflin JA, Hamasaki T, Freedman B (2009) Prevalence of physical signs in cervical myelopathy: a prospective, controlled study. Spine (Phila Pa 1976) 34(9):890–895
Al-Mefty O, Harkey LH, Middleton TH, Smith RR, Fox JL (1988) Myelopathic cervical spondylotic lesions demonstrated by magnetic resonance imaging. J Neurosurg 68(2):217–222
Tetreault L, Wilson JR, Kotter MR et al (2016) Predicting the minimum clinically important difference in patients undergoing surgery for the treatment of degenerative cervical myelopathy. Neurosurg Focus 40(6):E14
Arvin B, Kalsi-Ryan S, Mercier D et al (2013) Preoperative magnetic resonance imaging is associated with baseline neurological status and can predict postoperative recovery in patients with cervical spondylotic myelopathy. Spine (Phila Pa 1976) 38(14):1170–1176
Vedantam A, Jonathan A, Rajshekhar V (2011) Association of magnetic resonance imaging signal changes and outcome prediction after surgery for cervical spondylotic myelopathy. J Neurosurg Spine 15(6):660–666
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
NÖ, OG was involved in conceptualization and methodology; NÖ, OG, MC contributed to formal analysis and investigation; NÖ, OG, MC, TS were involved in writing—original draft preparation; NÖ, MC, TS, TFD, MH, AP, RJ, YA, US, NEH, OG contributed to writing—review and editing; US was involved in supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The study has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) and was approved by the Institutional Review Board (Medical Faculty of the University of Duisburg-Essen, registration number: 16–6270-BO).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Özkan, N., Chihi, M., Schoemberg, T. et al. First neurological symptoms in degenerative cervical myelopathy: does it predict the outcome?. Eur Spine J 31, 327–333 (2022). https://doi.org/10.1007/s00586-021-07060-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-021-07060-3