Abstract
Purpose
With lumbar laminectomy increasingly being performed on an outpatient basis, optimal pain management is critical to avoid post-operative delay in discharge and readmission. The aim of this review was to evaluate the available literature and develop recommendations for optimal pain management after one- or two-level lumbar laminectomy.
Methods
A systematic review utilizing the PROcedure-SPECific Post-operative Pain ManagemenT (PROSPECT) methodology was undertaken. Randomised controlled trials (RCTs) published in the English language from 1 January 2008 until 31 March 2020—assessing post-operative pain using analgesic, anaesthetic and surgical interventions—were identified from MEDLINE, EMBASE and Cochrane Databases.
Results
Out of 65 eligible studies identified, 39 RCTs met the inclusion criteria. The analgesic regimen for lumbar laminectomy should include paracetamol and a non-steroidal anti-inflammatory drug (NSAID) or cyclooxygenase (COX)—2 selective inhibitor administered preoperatively or intraoperatively and continued post-operatively, with post-operative opioids for rescue analgesia. In addition, surgical wound instillation or infiltration with local anaesthetics prior to wound closure is recommended. Some interventions—gabapentinoids and intrathecal opioid administration—although effective, carry significant risks and consequently were omitted from the recommendations. Other interventions were also not recommended because there was insufficient, inconsistent or lack of evidence.
Conclusion
Perioperative pain management for lumbar laminectomy should include paracetamol and NSAID- or COX-2-specific inhibitor, continued into the post-operative period, as well as intraoperative surgical wound instillation or infiltration. Opioids should be used as rescue medication post-operatively. Future studies are necessary to evaluate the efficacy of our recommendations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lumbar laminectomy is commonly performed in patients with lumbar spinal stenosis to relieve low back pain, reduce radiculopathy and improve overall function. These procedures are increasingly performed in an ambulatory or day-care setting. Inadequate pain management is one of the main reasons for delayed discharge or readmission after surgery [1, 4]. Effective pain control improves post-operative outcomes and patient satisfaction. Multimodal analgesia has frequently been recommended for enhanced recovery after surgery [5]. However, a lack of procedure-specific recommendations has resulted in heavy reliance on opioid medications [6]. Efforts to reduce opioid consumption and their associated adverse effects have been recently promoted [7].
The PROSPECT (PROcedure-SPECific Post-operative Pain ManagemenT) Working Group is a collaboration of surgeons and anaesthesiologists working to formulate procedure-specific recommendations for pain management after common but potentially painful operations. The recommendations are based on procedure-specific evidence as well as the balance between the efficacy and adverse effects of analgesic techniques and clinical relevance [8, 9].
The aim of this systematic review was to evaluate the available evidence on the management of pain after lumbar laminectomy. Post-operative pain outcomes (pain scores and analgesic requirements) were the primary focus, but other recovery outcomes—including adverse effects—were also assessed, when reported, and the limitations of the data were critically reviewed. The ultimate aim was to develop recommendations for pain management after laminectomy.
Methods
A systematic review of the literature associated with analgesia after lumbar laminectomy was conducted in accordance with the PROSPECT methodology [9]. The PROSPECT methodology requires that at least two RCTs are available to provide any guidance.
Research question
‘How can we enhance perioperative pain management in patients undergoing lumbar laminectomy?’.
Eligibility criteria
Inclusion criteria were randomised control trials (RCTs) or systematic reviews of analgesic, anaesthetic and operative interventions, published in the English language assessing pain management for patients undergoing up to two-level lumbar laminectomy. The study was required to measure pain intensity using tools such as the numerical rating scale or visual analogue scale. Studies that reported data pooled from patients undergoing mixed surgical procedures were excluded if no response was received from the authors to provide data tables specifically related to laminectomy and the intended intervention. Only open procedures were deemed eligible, and minimal invasive procedures were therefore excluded.
Search strategy
EMBASE, MEDLINE, Pubmed and Cochrane Databases (Cochrane Central Register of Controlled Trials, Cochrane Database of Abstracts or Reviews of Effects, Cochrane Database of Systematic Reviews) were searched for studies published between 1 January 2008 and 31 March 2020.
Search terms related to pain and interventions for laminectomy AND pain OR pains OR pain management OR post-operative pain OR post-operative pain OR analgesia* OR anaesthesia* OR vas OR visual analogue* OR vrs OR verbal rating scale* OR nrs OR numerical rating scale* OR pain rating OR epidural OR neuraxial OR intrathecal OR paravertebral OR spinal OR infiltration OR nerve block* OR neural block* OR paravertebral block* OR field block* OR Ilioinguinal block* OR transversus abdominis plane block* OR tap block* OR NSAID* OR non-steroidal anti-inflammatory* OR non-steroidal anti-inflammatory* OR COX-2 OR Paracetamol OR acetaminophen OR clonidine OR opioid* OR ketamine OR corticosteroid* OR gabapentin OR pregabalin.
Study selection
A stepwise manner was used, which included screening of abstracts of potential articles. This process was undertaken by two reviewers, and the final results of each reviewer were compared. Any discrepancies between results were discussed within the working group, and a decision was made on inclusion or exclusion by consensus.
Assessment of the quality of evidence
The final articles were assessed by all authors, and again any discrepancies were resolved in the same fashion. Reasons for exclusion were provided for all articles that were excluded in this phase. Reference lists of the relevant articles were individually screened to assess for any additional articles that may have been missed in the initial literature search. Criteria employed in the assessment of the quality of eligible studies (Supplementary Table 1) included allocation concealment (A–adequate; B–Unclear; C–inadequate; D–not used); the numerical (1–5) quality scoring system employed by Jadad to assess randomisation, double blinding and flow of patients; a participant follow-up of greater or less than 80 percent; and whether the study met the requirements of the Consolidated Standards of Reporting Trials (CONSORT) 2010 Statement.
Data extraction
Summary information for each included study was extracted and recorded in data tables. This information included timing of the intervention and mode of delivery, pain scores, whether pain was assessed at rest or during mobilisation, supplementary analgesic use, time to first analgesic administration, time intervals of pain measured. Unless specified otherwise, it was assumed that the pain scores were assessed at rest. The included studies were grouped together based upon the analgesic interventions (e.g. paracetamol, non-steroidal anti-inflammatory drugs [NSAIDs], gabapentinoids [pregabalin and gabapentin] and locoregional analgesia among others). The study assessing the effects of surgical techniques on analgesic outcomes was grouped separately.
Pain intensity scores were used as primary outcome measures. A difference in pain scores, between the intervention group and control group, of at least 1 cm/10 cm on VAS or 1/10 on NRS has been considered as clinically significant based on the publication by Myles et al. [10]. According to the PROSPECT methodology, clinically significant differences, rather than statistically significant differences, are used to determine recommendation. The effectiveness of each intervention for each outcome was evaluated qualitatively, by assessing the number of studies showing a significant difference between treatment arms (P < 0.05, as reported in the study publication). A meta-analysis was not performed due to the limited number of studies with homogenous design and differences in reporting of results, restricting pooled analysis.
Formulation of the recommendations
Recommendations are given when at least two congruent studies support an intervention. The proposed recommendations were sent to the PROSPECT Working Group for review and comments. A modified Delphi approach was utilised, which included several rounds of individual comments followed by round-table discussions. The modified Delphi method involved achieving consensus on recommendation for analgesic interventions that have at least two RCTs. Once consensus was achieved, the final manuscript was drafted by the lead authors, which was ultimately approved by the Working Group.
Results
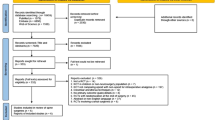
The PRISMA flowchart establishing the search strategy and data is presented in Fig. 1. The methodological quality assessments of the 39 RCTs included for the final qualitative analysis are summarised in Supplementary Table 1. The characteristics of the included studies are shown in Supplementary Tables 2, 3.
Systemic Analgesic Interventions
Cakan et al. performed a placebo-controlled trial to evaluate the effect of intravenous (IV) paracetamol 1000 mg during the first 24 post-operative hours [11]. Rescue analgesia included IV patient-controlled analgesia (IV-PCA) morphine. Pain scores were significantly lower at 12 h, 18 h and 24 h post-surgery. However, morphine consumption was not statistically significant between the groups.
Kesimci et al. compared oral dexketoprofen 25 mg to oral paracetamol 500 mg to placebo, 30 min before induction of anaesthesia [12]. Rescue analgesia included IV-PCA morphine. There were no significant differences in pain scores between groups; however, opioid consumption was significantly lower in the dexketoprofen group.
Khajavikhan et al. compared celecoxib 400 mg administered 2 h before surgery and 200 mg administered 6 h after surgery with placebo [13]. Rescue analgesia included intermittent IV morphine. Significantly lower pain scores were noted in the celecoxib group, and total opioid consumption was also significantly lower in the celecoxib group.
Attia et al. compared oral etoricoxib 120 mg, oral duloxetine 60 mg, the combination of oral etoricoxib 120 mg and duloxetine 60 mg and placebo [14]. The drugs were administered 1 h before surgery as well as 24 h after surgery. Rescue analgesia included IV paracetamol and intermittent IV morphine. Pain scores were significantly lower at all times in patients receiving the combination of etoricoxib and duloxetine as well in patients receiving etoricoxib alone. Patients receiving the combination of etoricoxib and duloxetine also had significantly lower opioid consumption after surgery.
Duttchen et al. compared IV ketorolac 30 mg to IV ketorolac 15 mg [15]. Rescue analgesia included intermittent IV morphine. There was no significant difference between the two groups.
Nikooseresh et al. compared diclofenac 100 mg suppository to IV paracetamol 1000 mg [16]. Rescue analgesia included IV-PCA fentanyl. There was no significant difference in pain scores; however, opioid consumption was significantly lower with diclofenac.
Cassinelli et al. compared ketorolac 30 mg (15 mg if patient age > 65 years) to placebo [17]. Rescue analgesia included oral oxycodone and intermittent IV morphine. Pain scores and opioid consumption were significantly lower at 0 h and 4 h after surgery in patients receiving ketorolac.
Emamhadi et al. compared diclofenac 100 mg suppository to IM pethidine 0.5 mg/kg [18]. Rescue analgesia was not reported. Significantly lower pain scores were reported with pethidine at all time points after surgery.
Yadav et al. compared pregabalin 150 mg to pregabalin 300 mg and to placebo, administered 2 h before surgery [19]. Rescue analgesia included oral NSAIDs and IV-PCA fentanyl. Pain scores and opioid consumption after surgery were significantly lower in both groups of patients receiving pregabalin, without significant differences between the two different doses of pregabalin. There was a higher incidence of dizziness and blurred vision in patients receiving pregabalin 300 mg.
Kumar et al. compared oral pregabalin 150 mg, administered 1 h before induction, with oral tramadol 100 mg and with placebo [20]. Rescue analgesia included intermittent IV fentanyl and IV diclofenac. Significantly lower pain scores and opioid consumption were seen with both pregabalin and tramadol. Post hoc analysis significantly favoured the tramadol group. No significant difference in anxiety was reported. Adverse effects were not reported.
Choi et al. compared oral pregabalin 150 mg—administered twice a day with a total of 8 doses—to the combination of oral pregabalin 150 mg and IV dexamethasone 5 mg and to placebo [21]. Rescue analgesia included continuous IV fentanyl. Pain scores were significantly lower in both intervention groups. Opioid consumption was significantly lower with the combination of pregabalin and dexamethasone. No significant differences in adverse effects were noted.
Javaherforooshzadeh et al. compared oral gabapentin 600 mg, administered 100 min before surgery, to oral melatonin 6 mg and to placebo [22]. Rescue analgesia included IV morphine and IV pethidine. Pain scores were significantly lower in patients that received gabapentin. Opioid consumption was significantly lower in both gabapentin and melatonin groups. No significant differences in adverse effects were noted.
Khan et al. compared oral gabapentin 600 mg, oral gabapentin 900 mg, oral gabapentin 1200 mg and placebo [23]. Rescue analgesia included IV-PCA morphine. Pain scores and opioid consumption were significantly lower in the gabapentin 900 mg and 1200 mg groups. The time of administration (2 h before surgery or at the end of surgery) did not impact the analgesic effect. Adverse effects were not reported.
Vasigh et al. compared oral gabapentin to oral celecoxib in two RCTs [24, 25]. Rescue analgesia included intermittent IV morphine. One RCT compared oral gabapentin 600 mg administered 2 h before surgery and 300 mg 6 h after surgery, the combination of oral gabapentin 300 mg and oral celecoxib 200 mg administered 2 h before surgery and 6 h after surgery and placebo [24]. Pain scores and opioid consumption were significantly lower in patients receiving the combination of gabapentin and celecoxib. The other RCT compared oral gabapentin 600 mg administered 2 h before surgery and 300 mg 6 h after surgery with oral celecoxib 400 mg administered 2 h before surgery and 200 mg 6 h after surgery and with placebo [25]. Pain scores were lower in patients receiving gabapentin, and opioid consumption was significantly lower in both intervention groups. Adverse effects were not reported.
Ozgencil et al. compared oral pregabalin 150 mg, oral gabapentin 1200 mg and placebo, administered twice before surgery and twice after surgery [26]. Rescue analgesia included IV-PCA morphine. Pain scores were significantly lower with pregabalin and gabapentin at 1 h, 2 h, 4 h and 6 h after surgery. Opioid consumption was significantly lower in both gabapentin and pregabalin groups at all time points, except at 6 h after surgery where opioid consumption was lower with pregabalin. Except for pruritus, the adverse effects observed were similar among groups. The incidence of pruritus was lower in both the gabapentin and pregabalin groups compared to the placebo group.
Wittayapairoj et al. compared IV dexamethasone 0.2 mg/kg administered before surgery to placebo [27]. Rescue analgesia included IV-PCA morphine. Pain scores were not significantly different between the two groups, but opioid consumption was significantly lower in patients receiving dexamethasone.
Ghaffaripour et al. compared IV magnesium, with a loading dose of 30 mg/kg at the start of surgery and a continuous infusion of 10 mg/kg/h during surgery, to placebo [28]. Rescue analgesia included IV-PCA morphine. There were no significant differences between the two groups.
Esmat et al. compared a transdermal fentanyl patch (50 µg/u), a transdermal melatonin delivery system (7 mg) and a transdermal placebo patch [29]. Rescue analgesia included IM pethidine. Pain scores did not differ significantly between groups, but opioid consumption was lower with transdermal fentanyl and melatonin.
Locoregional anaesthesia
Chan et al. evaluated the analgesic effects of intrathecal fentanyl 15 µg [30]. Patients in the control group did not receive an intervention. Rescue analgesia included IV-PCA morphine. Pain scores and opioid consumption were significantly lower in patients receiving intrathecal fentanyl. No significant differences in adverse effects were noted.
Yen et al. compared intrathecal morphine 3.5 µg/kg (with a maximum dose of 350 µg) to placebo [31]. Rescue analgesia included IV-PCA morphine. There was no significant difference in pain scores. Total opioid consumption, however, was significantly lower in patients receiving intrathecal morphine. No episodes of respiratory depression were observed in both groups.
Firouzian et al. compared the intrathecal morphine 200 µg to the combination of intrathecal morphine 200 µg and naloxone 20 µg [32]. Rescue analgesia included IV-PCA morphine. Pain scores and opioid consumption were significantly lower in patients receiving the combination of intrathecal morphine and naloxone. No significant differences in adverse effects were noted.
Kundra et al. compared epidural gelfoam soaked in morphine 5 mg to the combination of epidural gelfoam soaked in saline and epidural instillation with morphine 5 mg [33]. Rescue analgesia included IV diclofenac and intermittent IV morphine. Pain scores and opioid consumption were significantly lower in patients receiving epidural gelfoam soaked in morphine 5 mg. No significant differences in adverse effects were noted.
Guilfoyle et al. evaluated the analgesic effects of fentanyl 100 µg administered through an epidural catheter [34]. Patients in the control group received no intervention. Rescue analgesia was not reported. Pain scores were significantly lower in patients that received epidural fentanyl when admitted to the recovery, but not afterwards. No significant differences in adverse effects were noted.
Hassanein et al. compared epidural gelfoam soaked in morphine 5 mg (diluted in crystalloid), epidural gelfoam soaked in morphine 5 mg (diluted in colloid) and epidural instillation with morphine 5 mg [35]. Rescue analgesia included IV diclofenac and intermittent IV morphine. Pain scores and opioid consumption were significantly lower in both epidural gelfoam groups. No significant differences in adverse effects were noted.
Kumari et al. compared epidural gelfoam soaked in 10 ml levobupivacaine 0.25% combined with dexamethasone 10 mg, epidural gelfoam soaked in 10 ml levobupivacaine 0.25% combined with saline and epidural gelfoam soaked in saline only [36]. Rescue analgesia included IV tramadol. Pain scores and opioid consumption were significantly lower in both groups that received epidural gelfoam soaked in levobupivacaine. The addition of dexamethasone did not result in significant differences. No significant differences in adverse effects were noted.
Giri et al. compared epidural gelfoam soaked in ketamine 50 mg diluted with 5 mL saline, epidural gelfoam soaked in nalbuphine 10 mg diluted with 5 mL saline and epidural gelfoam soaked in 5 mL saline [37]. Rescue analgesia included IV diclofenac. Pain scores were significantly lower in both intervention groups. Total diclofenac consumption was significantly lower in patients receiving epidural gelfoam soaked in ketamine 50 mg. No significant differences in adverse effects were noted.
Ozbek et al. evaluated the analgesic effects of a paravertebral block, performed with 5 mL levobupivacaine 0.5% for each nerve to upper dermatome of laminectomy level [38]. Patients in the control group did not receive any intervention. Rescue analgesia included IV-PCA morphine. Pain scores and opioid consumption were significantly lower in patients receiving a paravertebral block.
Mordeniz et al. evaluated the analgesic effects of a perineural infiltration with 2 ml of bupivacaine 0.5% [39]. Perineural infiltration was defined as the infiltration of local anaesthetics in the irritated neural root sheath, before root extraction. Patients in the control group did not receive any intervention. Rescue analgesia included IV tramadol Opioid consumption after surgery was significantly lower in patients that received a perineural infiltration.
Torun et al. evaluated the analgesic effects of a perineural infiltration with 0.5 ml of lidocaine 2% [40]. Patients in the control group did not receive any intervention. Rescue analgesia included IV tramadol. Opioid consumption after surgery was significantly lower in patients that received a perineural infiltration.
Saini et al. compared wound instillation with 20 ml of ropivacaine 0.25% to placebo [41]. Wound instillation was defined as the irrigation of the local analgesic into the surgical area for a dwell time of 60 s. Rescue analgesia included IV paracetamol and IV diclofenac. Pain scores and opioid consumption after surgery were significantly lower in the intervention group.
Jonnavithula et al. compared wound instillation with 20 ml of bupivacaine 0.25% to placebo [42]. Rescue analgesia included IM diclofenac. Pain scores and opioid consumption were significantly lower in patients that received wound instillation with bupivacaine.
Rahmanian et al. compared surgical wound instillation with 30 ml of bupivacaine 0.25% with placebo [43]. Rescue analgesia was not reported. Pain scores after surgery did not differ between the two groups.
Gurbet et al. compared wound infiltration with 20 ml of levobupivacaine 0.25% combined with methylprednisolone 40 mg, wound infiltration with 20 ml of bupivacaine 0.25% combined with methylprednisolone 40 mg and placebo [44]. Wound infiltration was defined as direct administration of the local analgesic along the line of the incision. Rescue analgesia included IV-PCA morphine. Pain scores and opioid consumption were significantly lower in both intervention groups.
Hazarika et al. compared local wound infiltration with 20 ml of bupivacaine 0.25% combined with magnesium sulphate 500 mg to 20 ml of ropivacaine 0.25% combined with magnesium sulphate 500 mg [45]. Rescue analgesia included IV nalbuphine. There was no significant difference in pain scores after surgery; however, opioid consumption was significantly lower in patients that received local wound infiltration with bupivacaine.
Multimodal analgesia
Garcia et al. evaluated the analgesic effects of a multimodal analgesic regimen (celecoxib 100 mg twice a day, pregabalin 75 mg twice a day and oxycodone 10 mg twice a day) [46]. Patients in the control group did not receive any intervention. Rescue analgesia included intermittent IV morphine. Pain scores and opioid consumption were significantly lower at all time points in patients receiving the multimodal regimen.
Anaesthetic technique
Vasigh et al. compared induction of anaesthesia with thiopentone and maintenance with sevoflurane, induction and maintenance with propofol, and induction with propofol and maintenance with sevoflurane [47]. Rescue analgesia included intermittent IV morphine. Pain scores and opioid consumption were significantly lower with an induction of anaesthesia with propofol and maintenance with sevoflurane.
Duger et al. compared spinal anaesthesia with 2 ml of bupivacaine 0.5% combined with morphine 0.1 mg, epidural anaesthesia with 10 ml of bupivacaine 0.5% combined with morphine 2 mg and combined spinal and epidural anaesthesia [CSE] with 1 ml of intrathecal bupivacaine 0.5% combined with morphine 0.05 mg and 6 ml of epidural bupivacaine 0.5% combined with morphine 2 mg [48]. Rescue analgesia included IV-PCA morphine. Pain scores and opioid consumption were significantly lower with epidural anaesthesia and CSE.
Surgical technique
Watanabe et al. compared the technique of lumbar spinous process splitting laminectomy (LSPSL) to the conventional technique of laminectomy [49]. Rescue analgesia included oral NSAIDs. Pain scores were significantly lower with LSPSL. There was no significant difference between groups in opioid consumption.
Discussion
Interpretation
This systematic review included 39 RCTs with the majority of studies determined to be of high quality by the CONSORT statement. The strength of our systematic review stems from the PROSPECT methodology which goes beyond making recommendation based on the simple statistical analysis of the available evidence. The included studies are interpreted based on the use of a baseline analgesic technique (i.e. use of paracetamol and NSAID- or COX-2-specific inhibitor) and balancing the efficacy and adverse effects of the intervention as well as assimilating this information in the perioperative setting of lumbar laminectomy. Overall, the PROSPECT recommendations provide clinicians with supporting arguments for and against the use of analgesic interventions for laminectomy.
Based on the PROSPECT methodology, a combination of paracetamol and NSAID- or COX-2-specific inhibitor is recommended preoperatively or intraoperatively, which should be continued into the post-operative period, unless there are contraindications. Excellent evidence is available for the use of NSAIDs/COX-2-specific inhibitors, with five out of seven RCTs demonstrating improved outcomes.[12,13,14, 16, 17]. Duttchen et al. could not demonstrate a significant difference in outcomes between two different doses of ketorolac [15]. Emamhadi et al. compared the use of diclofenac to pethidine, showing less pain in patients treated with pethidine [18]. On the other hand, there is only one RCT available where the effect of paracetamol in patients undergoing lumbar laminectomy was evaluated [11]. However, the opioid‐sparing effects of paracetamol are well described, and for this reason, paracetamol is also recommended for lumbar laminectomy. Furthermore, both Kesimci et al. and Nikosereth et al. reported no significant difference in post-operative pain scores between NSAIDs and paracetamol [12, 16].
Wound instillation or infiltration with local anaesthetics, performed by the surgeon just before wound closure, is recommended as the regional anaesthetic technique of choice. Wound instillation is defined as the irrigation of the local anaesthetics into the surgical area, while wound infiltration is defined as the direct injection of local anaesthetics into the tissue along the line of incision. For this recommendation, we decided not to distinguish between wound instillation and wound infiltration because these two techniques are closely related. Three RCTs of high quality showed significantly improved outcomes using wound instillation or infiltration [41, 42, 44]. One RCT did not show a significant analgesic benefit after wound instillation [43]. Another RCT compared the infiltration of two different local anaesthetics without a placebo group [45]. It is possible that some of the benefits from local anaesthetic wound instillation may be due to migration of local anaesthetic into the neuraxial planes. Surgical wound instillation or infiltration remains a simple technique that can be rapidly performed, with limited risk for side effects including anaesthetic systemic toxicity. Although the studies did not describe infiltration techniques, it is well accepted that any surgical site infiltration should involve local anaesthetic injection into multiple layers, similar to local infiltration techniques used for joint surgery. Intrathecal opioids have been demonstrated to provide excellent pain relief in patients undergoing lumbar laminectomy [30, 32]. However, the potential side effects are worrisome, particularly because this procedure is increasingly being performed as an outpatient procedure [2, 3]. These potential side effects include—but are not limited to—respiratory depression, cardiovascular stress, cognitive dysfunction, delayed wound healing, urinary and gastrointestinal dysfunction, as well as the risk of acquired tolerance and long-term opioid use. Therefore, it is prudent to avoid intrathecal opioids. In addition, it is unclear whether these neuraxial techniques provide any further enhanced clinically relevant pain relief over the use of basic analgesics combined with local anaesthetic instillation or infiltration. For the same reason, epidural analgesia is not recommended [33, 37]. Other regional anaesthetic techniques, such as paravertebral blocks and perineural infiltration, are not recommended due to limited procedure-specific evidence [38, 40].
Gabapentinoids are not recommended as the first line of treatment in spite of proven efficacy in this patient population [19, 26]. Similar to intrathecal and epidural analgesia, a significant risk of important side effects (including, but not limited to sedation, dizziness, visual blurring) is associated with the administration of these drugs. The FDA recently published an advisory emphasising the concerns of gabapentin and pregabalin [50].
Only limited procedure-specific evidence was available for dexamethasone, with one RCT showing reduced opioid consumption [27]. Therefore, dexamethasone cannot be recommended as part of the standard analgesic regimen in patients undergoing lumbar laminectomy. Nonetheless, it has an important role in the prevention of post-operative nausea and vomiting [51]. There were no published data assessing the analgesic effects of ketamine and alpha-2-agonists, such as dexmedetomidine or clonidine, which could be assessed in future studies.
We have found no evidence regarding analgesic regimens for challenging patient populations, such as chronic opioid consumers, which is a common phenomenon in this surgical population. Hence, there is a need for further research on this topic.
Opioids are recommended only as rescue medication for patients undergoing lumbar laminectomy [52]. We caution against the use of transdermal fentanyl patches in the perioperative period, because this treatment is not adapted for the treatment of acute post-operative pain [53].
Limitations
The limitations in this review are related to those of the included studies. There was considerable heterogeneity between studies such as variable dosing regimens, variable methods of administration and variable control groups as well as variable time points of pain assessments. The small size of most studies has the potential for estimation effect. In addition, the sample size of the studies was not adequate to draw valid conclusions concerning the safety profile of the analgesic interventions. Also, the analgesic interventions were not evaluated against a control group that included an optimised multimodal analgesic regimen.
Implications for future research
Future adequately powered studies should assess the effects of analgesic interventions not only on pain, opioid consumption, opioid-related adverse events and complications associated with the intervention, but also outcome measures such as time to ambulation, length of hospital stay and the occurrence of chronic pain or chronic opioid consumption. Furthermore, the influence of analgesic intervention on patient-specific factors such as chronic pain and chronic opioid therapy needs to be assessed.
Conclusion
In summary, this review has identified a perioperative analgesic regimen for optimal pain management after lumbar laminectomy (Table 1). This review also identified perioperative interventions that are not recommended for pain management in patients undergoing lumbar laminectomy (Table 2). Perioperative pain management for lumbar laminectomy should include paracetamol and NSAID- or COX-2-specific inhibitor, continued into the post-operative period, as well as intraoperative surgical wound instillation or infiltration. Opioids should be used as rescue medication post-operatively.
References
Elsharydah A, Duncan KL, Rosero EB et al (2020) Readmission rate after 2-level lumbar decompression: a propensity-matched cohort study comparing inpatient and outpatient settings. Clin Spine Surg. https://doi.org/10.1097/BSD.0000000000000990
Pendharkar AV, Shahin MN, Ho AL et al (2018) Outpatient spine surgery: defining the outcomes, value, and barriers to implementation. Neurosurg Focus 44(5):E11. https://doi.org/10.3171/2018.2.FOCUS17790
Yen D, Albargi A (2017) Results and limitations of outpatient and overnight stay laminectomies for lumbar spinal stenosis. Can J Surg 60(5):329–334. https://doi.org/10.1503/cjs.002017
Mundell BF, Gates MJ, Kerezoudis P et al (2018) Does patient selection account for the perceived cost savings in outpatient spine surgery? A meta-analysis of current evidence and analysis from an administrative database. J Neurosurg Spine 29(6):687–695. https://doi.org/10.3171/2018.4.SPINE1864
Joshi GP, Kehlet H (2019) Postoperative pain management in the era of ERAS: an overview. Best Pract Res Clin Anaesthesiol 33(3):259–267. https://doi.org/10.1016/j.bpa.2019.07.016
Kurd MF, Kreitz T, Schroeder G, Vaccaro AR (2017) The Role of Multimodal Analgesia in Spine Surgery. J Am Acad Orthop Surg 25(4):260–268. https://doi.org/10.5435/JAAOS-D-16-00049
Dietz N, Sharma M, Adams S et al (2019) Enhanced recovery after surgery (ERAS) for Spine surgery: a systematic review. World Neurosurg 130:415–426. https://doi.org/10.3171/2019.1.FOCUS18700
Joshi GP, Kehlet H (2017) Prospect working group guidelines for perioperative pain management need for re-evaluation. Br J Anaesth 119(4):703–706. https://doi.org/10.1093/bja/aex304
Joshi GP, Van de Velde M, Kehlet H (2019) Prospect working group collaborators. Development of evidence-based recommendations for procedure-specific pain management: prospect methodology. Anaesthesia 74(10):1298–1304. https://doi.org/10.1111/anae.14776
Myles PS, Myles DB, Galagher W, Boyd D, Chew C, MacDonald N, Dennis A (2017) Measuring acute postoperative pain using the visual analog scale: the minimal clinically important difference and patient acceptable symptom state. Br J Anaesth 118(3):424–429. https://doi.org/10.1093/bja/aew466
Cakan T, Inan N, Culhaoglu S, Bakkal K, Başar H (2008) Intravenous paracetamol improves the quality of postoperative analgesia but does not decrease narcotic requirements. J Neurosurg Anesthesiol 20(3):169–173. https://doi.org/10.1097/ANA.0b013e3181705cfb
Kesimci E, Gümüş T, Izdeş S, Sen P, Kanbak O (2011) Comparison of efficacy of dexketoprofen versus paracetamol on postoperative pain and morphine consumption in laminectomy patients. Agri 23(4):153–159. https://doi.org/10.5505/agri.2011.86548
Khajavikhan J, Aminolah V, Molouk J, Ali K (2016) A multimodal analgesia of Cyclooxygenase-2 for postoperative pain. Der Pharmacia Lettre 8:195–202
Attia JZ, Mansour HS (2017) Perioperative Duloxetine and Etoricoxibto improve postoperative pain after lumbar Laminectomy: a randomized, double-blind, controlled study. BMC Anesthesiol 17(1):162. https://doi.org/10.1186/s12871-017-0450-z
Duttchen KM, Lo A, Walker A et al (2017) Intraoperative ketorolac dose of 15 mg versus the standard 30 mg on early postoperative pain after spine surgery: a randomized, blinded, non-inferiority trial. J Clin Anesth 41:11–15. https://doi.org/10.1016/j.jclinane.2017.05.013
Nikooseresht M, Seifrabiei MA, Davoodi M, Aghajanlou M, Sardari MT (2016) Diclofenac suppository versus IV Acetaminophen combined with IV PCA for postoperative pain management in patients undergoing laminectomy a randomized double-blinded clinical trial. Anesth Pain Med 6(3):e36812. https://doi.org/10.5812/aapm.36812
Cassinelli EH, Dean CL, Garcia RM, Furey CG, Bohlman HH (2008) Ketorolac use for postoperative pain management following lumbar decompression surgery: a prospective, randomized, double-blinded, placebo-controlled trial. Spine 33(12):1313–1317. https://doi.org/10.1097/BRS.0b013e31817329bd
Emamhadi MR, Hatamian HR (2016) Comparing the effect of intramuscular injection of pethidine and diclofenac suppository in relief of pain following laminectomy surgery. Acta Medica Iranica 46:287–290
Yadav R, Mishra RK, Chaturvedi A, Rath GP (2018) Effect of pregabalin on preoperative anxiety and postoperative pain in spine surgery: a randomized controlled trial. J Neuroanaesthesiol Crit Care 5:8–14. https://doi.org/10.1055/s-0037-1616037
Kumar KP, Kulkarni DK, Gurajala I, Gopinath R (2013) Pregabalin versus tramadol for postoperative pain management in patients undergoing lumbar laminectomy: a randomized, double-blinded, placebo-controlled study. J Pain Res 6:471–478. https://doi.org/10.2147/JPR.S43613
Choi YS, Shim JK, Song JW, Kim JC, Yoo YC, Kwak YL (2013) Combination of pregabalin and dexamethasone for postoperative pain and functional outcome in patients undergoing lumbar spinal surgery: a randomized placebo-controlled trial. Clin J Pain 29(1):9–14. https://doi.org/10.1097/AJP.0b013e318246d1a9
Javaherforooshzadeh F, Amirpour I, Janatmakan F, Soltanzadeh M (2018) Comparison of effects of melatonin and gabapentin on post operative anxiety and pain in lumbar Spine surgery: a randomized clinical trial. Anesth Pain Med 8(3):e68763. https://doi.org/10.5812/aapm.68763
Khan ZH, Rahimi M, Makarem J, Khan RH (2011) Optimal dose of pre-incision/post-incision gabapentin for pain relief following lumbar laminectomy: a randomized study. Acta Anaesthesiol Scand 55(3):306–312. https://doi.org/10.1111/j.1399-6576.2010.02377.x
Vasigh A, Jaafarpour M, Khajavikhan J, Khani A (2016) The Effect of Gabapentin Plus Celecoxib on Pain and Associated Complications After Laminectomy. J Clin Diagn Res 10(3):UC04-UC8. https://doi.org/10.7860/JCDR/2016/17923.7346
Vasigh A, Najafi F, Khajavikhan J, Jaafarpour M, Khani A (2016) Comparing gabapentin and celecoxib in pain management and complications after laminectomy: a randomized double-blind clinical trial. Iran Red Crescent Med J 18(2):e34559. https://doi.org/10.5812/ircmj.34559
Ozgencil E, Yalcin S, Tuna H, Yorukoglu D, Kecik Y (2011) Perioperative administration of gabapentin 1,200 mg day-1 and pregabalin 300 mg day-1 for pain following lumbar laminectomy and discectomy: a randomised, double-blinded, placebo-controlled study. Singapore Med J 52(12):883–889
Wittayapairoj A, Wittayapairoj K, Kulawong A, Huntula Y (2017) Effect of intermediate dose dexamethasone on post-operative pain in lumbar spine surgery: a randomized, triple-blind, placebo-controlled trial. Asian J Anesthesiol 55(3):73–77. https://doi.org/10.1016/j.aja.2017.08.001
Ghaffaripour S, Mahmoudi H, Eghbal H, Rahimi A (2016) The effect of intravenous magnesium sulfate on post-operative analgesia during laminectomy. Cureus 8(6):e626. https://doi.org/10.7759/cureus.626
Esmat IM, Kassim DY (2016) Comparative study between transdermal fentanyl and melatonin patches on postoperative pain relief after lumbar laminectomy, a double-blind, placebo-controlled trial. Egypt J Anaesth 32:323–332. https://doi.org/10.1016/j.egja.2016.04.001
Chan JH, Heilpern GN, Packham I et al (2006) A prospective randomized double-blind trial of the use of intrathecal fentanyl in patients undergoing lumbar spinal surgery. Spine 31(22):2529–2533. https://doi.org/10.1097/01.brs.0000241135.79983.52
Yen D, Turner K, Mark D (2015) Is a single low dose of intrathecal morphine a useful adjunct to patient-controlled analgesia for postoperative pain control following lumbar spine surgery? A preliminary report. Pain Res Manag 20(3):129–132. https://doi.org/10.1155/2015/761390
Firouzian A, Gholipour Baradari A, Ehteshami S et al (2020) The effect of ultra-low-dose intrathecal naloxone on pain intensity after lumbar laminectomy with Spinal fusion: a randomized controlled trial. J Neurosurg Anesthesiol 32(1):70–76. https://doi.org/10.1097/ANA.0000000000000537
Kundra S, Gupta V, Bansal H et al (2014) Comparative study of epidural application of morphine versus gelfoam soaked in morphine for lumbar laminectomy. J Anaesthesiol Clin Pharmacol 30(1):46–52. https://doi.org/10.4103/0970-9185.125703
Guilfoyle MR, Mannion RJ, Mitchell P, Thomson S (2012) Epidural fentanyl for postoperative analgesia after lumbar canal decompression: a randomized controlled trial. Spine J 12(8):646–651. https://doi.org/10.1016/j.spinee.2012.07.007
Hassanein A, Ali NS, Saad A (2016) Colloid versus crystalloid soaked gelfoam with morphine for postoperative pain relief after lumbar laminectomy. Egypt J Anesth 32:495–502. https://doi.org/10.1016/j.egja.2016.08.019
Kumari K, Kamal M, Singariya G et al (2018) Effect of epidural levobupivacaine with or without dexamethasone soaked in gelfoam for postoperative analgesia after lumbar laminectomy: a double blind, randomised, controlled trial. Indian J Anaesth 62(7):509–515. https://doi.org/10.4103/ija.IJA_128_18
Giri MK, Singh V, Pal P, Mishra LS, Gopal NN (2018) A prospective randomized comparative study of gelfoam soaked nalbuphine versus ketamine placed in epidural space during lumber spine surgery for postoperative analgesia. Anaesth Pain Intensive Care 22(4):492–498
Ozbek TH, Gedik YE, Gunes Y, Yilmaz D, Isik G (2009) The analgesic effect of preemptive lumbar paravertebral block in patients undergoing laminectomy. Neurosurg Quart 19:160–163. https://doi.org/10.1097/WNQ.0b013e3181a45ae3
Mordeniz C, Torun F, Soran AF et al (2010) The effects of pre-emptive analgesia with bupivacaine on acute post-laminectomy pain. Arch Orthop Trauma Surg 130(2):205–208. https://doi.org/10.1007/s00402-009-0961-2
Torun F, Mordeniz C, Baysal Z et al (2010) Intraoperative perineural infiltration of lidocaine for acute postlaminectomy pain: preemptive analgesia in spinal surgery. J Spinal Disord Tech 23(1):43–46. https://doi.org/10.1097/BSD.0b013e318198793c
Saini D, Yadav U (2018) Study of wound instillation technique for effective postoperative analgesia using ropivacaine in lumbar spine surgery. Anesth Essays Res 12(3):685–689. https://doi.org/10.4103/aer.AER_87_18
Jonnavithula N, Garre S, Pasupuleti S et al (2015) Wound instillation of local anesthetic bupivacaine for postoperative analgesia following lumbar laminectomy. Middle East J Anaesthesiol 23(2):193–198
Rahmanian A, Malekpour F, Rakei SM, Ghaffarpasand F, Mehrabani G (2016) The effects of bupivacaine on postoperative back pain after lumbar laminectomy: a randomized clinical trial. Neurosurg Quart 26:293–297. https://doi.org/10.1097/WNQ.0000000000000185
Gurbet A, Bekar A, Bilgin H, Ozdemir N, Kuytu T (2014) Preemptive wound infiltration in lumbar laminectomy for postoperative pain: comparison of bupivacaine and levobupivacaine. Turk Neurosurg 24(1):48–53. https://doi.org/10.5137/1019-5149.JTN.8431-13.0
Hazarika R, Parua S, Choudhury D, Barooah RK (2017) Comparison of bupivacaine plus magnesium sulfate and ropivacaine plus magnesium sulfate infiltration for postoperative analgesia in patients undergoing lumbar laminectomy: a randomized double-blinded study. Anesth Essays Res 11(3):686–691. https://doi.org/10.4103/0259-1162.206859
Garcia RM, Cassinelli EH, Messerschmitt PJ, Furey CG, Bohlman HH (2013) A multimodal approach for postoperative pain management after lumbar decompression surgery: a prospective, randomized study. J Spinal Disord Tech 26(6):291–297. https://doi.org/10.1097/BSD.0b013e318246b0a6
Vasigh A, Najafi F, Jaafarpour M, Khajavikhan J, Khani A (2017) The effect of sevoflurane plus propofol on pain and complications after laminectomy: a randomized double blind clinical trial. J Clin Diagn Res 11(4):05–08. https://doi.org/10.7860/JCDR/2017/23565.9643
Düger C, Gürsoy S, Karadağ O et al (2012) Anesthetic and analgesic effects in patients undergoing a lumbar laminectomy of spinal, epidural or a combined spinal-epidural block with the addition of morphine. J Clin Neurosci 19(3):406–410. https://doi.org/10.1016/j.jocn.2011.04.042
Watanabe K, Matsumoto M, Ikegami T et al (2011) Reduced postoperative wound pain after lumbar spinous process-splitting laminectomy for lumbar canal stenosis: a randomized controlled study. J Neurosurg Spine 14(1):51–58. https://doi.org/10.3171/2010.9.SPINE09933
FDA (2019) FDA warns about serious breathing problems with seizure and nerve pain medicines gabapentin (Neurontin, Gralise, Horizant) and pregabalin (Lyrica, Lyrica CR). Internet Document FDA.gov Available from: URL: https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-about-serious-breathing-problems-seizure-and-nerve-pain-medicines-gabapentin-neurontin. Accessed 10 Apr 2020
Gan TJ, Diemunsch P, Habib AS et al (2014) Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg 118:85–113. https://doi.org/10.1213/ANE.0000000000000002
Alexander JC, Patel B, Joshi GP (2019) Perioperative use of opioids: current controversies and concerns. Best Pract Res Clin Anaesthesiol 33:341–351. https://doi.org/10.1016/j.bpa.2019.07.009
Kaye AD, Menard BL, Ehrhardt KP et al (2019) Consensus perioperative management best practices for patients on transdermal fentanyl patches undergoing surgery. Curr Pain Headache Rep 23(7):50. https://doi.org/10.1007/s11916-019-0780-2
Acknowledgements
The authors want to thank the other members of the prospect group for their collaboration on this manuscript: G. P. Joshi, E. Pogatzki-Zahn, M. Van de Velde, S. Schug, H. Kehlet, F. Bonnet, N. Rawal, A. Delbos, P. Lavand’homme, H. Beloeil, J. Raeder, A. R. Sauter, E. Albrecht, P. Lirk, S. Freysand D. Lobo.
Funding
PROSPECT is supported by an unrestricted grant from the European Society of Regional Anaesthesia and Pain Therapy (ESRA). In the past, PROSPECT had received unrestricted grants from Pfizer Inc. New York, NY, USA, and Grunenthal, Aachen, Germany.
Author information
Authors and Affiliations
Consortia
Contributions
LP and PLC conducted the literature search and analysed the retrieved articles with AS, JG and HB. LP and PLC wrote the manuscript, which was reviewed and edited by all the other authors who have also participated in the PROSPECT Working Group meetings using the Delphi method and in defining the methodology of the PROSPECT group.
Corresponding author
Ethics declarations
Conflict of interest
Philipp Lirk has no conflicts of interest to declare. Girish P. Joshi has received honoraria from Baxter and Pacira Pharmaceuticals. Francis Bonnet has received honoraria from Pfizer, The Medicine Company, Abbott France and Nordic Pharma France. Henrik Kehlet has received honoraria from Pfizer and Grunenthal. The Anesthesiology Unit of the University of Western Australia, but not Stephan Schug privately, has received research and travel funding and speaking and consulting honoraria from bioCSL, Eli Lilly, Indivior, iX Biopharma and Pfizer. Narinder Rawal has received honoraria from Baxter and Sintetica. Marc Van de Velde received honoraria from Sintetica, Grunenthal, Vifor Pharma, MSD, Nordic Pharma, Janssen Pharmaceuticals, Heron Therapeutics and Aquettant. Hélène Beloeil has received honoraria from Orion, Abbvie and Aspen.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Peene, L., Le Cacheux, P., Sauter, A.R. et al. Pain management after laminectomy: a systematic review and procedure-specific post-operative pain management (prospect) recommendations. Eur Spine J 30, 2925–2935 (2021). https://doi.org/10.1007/s00586-020-06661-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-020-06661-8