Abstract
Purpose
A prospective, single-arm, open-label study to evaluate the effectiveness of intraosseous radio frequency (RF) ablation of the basivertebral nerve (BVN) for the treatment of vertebrogenic-related chronic low back pain (CLBP) in typical spine practice settings using permissive criteria for study inclusion.
Methods
Consecutive patients with CLBP of at least 6 months duration and with Modic Type 1 or 2 vertebral endplate changes between L3 and S1 were treated with RF ablation of the BVN in up to four vertebral bodies. The primary endpoint was patient-reported change in Oswestry Disability Index (ODI) from baseline to 3 months post-procedure. Secondary outcome measures included change in visual analog scale (VAS), SF-36, EQ-5D-5L, and responder rates.
Results
Median age was 45 years; baseline ODI was 48.5; VAS was 6.36. Seventy-five percent (75%) of the study patients reported LBP symptoms for ≥ 5 years; 25% were actively using opioids; and 61% were previously treated with injections. Mean change in ODI at 3 months posttreatment was − 30.07 +14.52 points (p < 0.0001); mean change in VAS was − 3.50 + 2.33 (p < 0.0001). Ninety-three percent (93%) of patients achieved a ≥ 10-point improvement in ODI, and 75% reported ≥ 20-point improvement.
Conclusions
Minimally invasive RF ablation of the BVN demonstrated a significant improvement in pain and function in this population of real-world patients with chronic vertebrogenic-related LBP.
Graphical abstract
These slides can be retrieved under Electronic Supplementary Material.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic LBP affects more than 30 million U.S. adults [1, 2]. While nonoperative management is often successful, recurrence is common and long-term disability has been reported in up to 17% [3]. Rates of traditional surgical intervention, i.e., fusion, and reported outcomes are highly heterogeneous [4].
Recently, studies have demonstrated that vertebrogenic pain from degenerated or damaged vertebral endplates is an important source of CLBP. The endplates are susceptible to damage due to their conflicting roles of providing nutritional support for the poorly vascularized disk and structural support for the spine. Factors implicated in symptomatic endplate failure include mechanical loading, local endplate morphology, and genetic predisposition [5, 6]. Endplate damage can lead to cellular communication between the immunologically privileged disk nucleus and vertebral bone marrow, triggering an inflammation—a process that can lead to Modic changes visible on MRI [7, 8].
The specific mechanisms for vertebrogenic pain have been detailed in immunohistochemical studies demonstrating endplate nociceptors with efferents to the basivertebral nerve (BVN), a branch of the sinuvertebral nerve located posteriorly in the vertebral body [6, 8, 9]. Chronic inflammation leads to endplate nociceptor proliferation. In the presence of chemical sensitization and mechanical stimulation, pain signals, perceived as LBP, are transmitted to the central nervous system by the BVN [6].
An improved understanding of this pathway generated interest in RF ablation of the BVN as a treatment for vertebrogenic CLBP. To date, clinical findings have been promising. An initial pilot study led to a multi-center, randomized sham-controlled trial [10]. First reported in 2018, the RCT demonstrated the efficacy of intraosseous RF ablation of the BVN to treat CLBP in patients with Modic type 1 or 2 changes of the vertebral endplates [11]. Sustained clinical improvement at 2-year follow-up has recently been reported [12].
Yet, novel spine care treatment modalities have been introduced with initially very promising results with later studies reporting poorer outcomes or greater complication rates than originally anticipated. Common reasons for this disappointing change lie in different patient populations, less rigid selection criteria, and the overall differences in treatment effectiveness (measures how well a treatment works in the practice of medicine) versus efficacy (measures how well treatment works in clinical trials or laboratory studies) [13, 14]. We sought to perform a prospective clinical effectiveness trial emulating a typical spine practice setting and employing more permissive criteria for study inclusion such as patients who have had prior discectomy and utilizers of extended-release narcotics.
Methods
Trial design
The RF ablation in CLBP study is a prospective, open-label, single-arm case series of 28 patients treated at two investigative sites in the USA from to June 2018 to January 2019. The study is registered on ClinicalTrials.gov as NCT03266107 and was sponsored by Relievant Medsystems, Inc. (Minneapolis, MN). The study was HIPAA compliant and conducted under Institutional Board Review approval, and participant informed consent was obtained. Enrolled patients were assigned a unique participant ID number, and all submitted patient information was de-identified. Data submitted by the study sites were source-verified to the medical record and patient-completed questionnaires by study monitors.
Participants
Eligibility was confirmed by an independent medical monitor based on each patient’s medical, clinical, and radiographic presentation. The primary inclusion criteria included skeletally mature patients with Type 1 or Type 2 Modic changes on an MRI at one or more vertebral bodies from L3 through S1 and ≥ 6 months of conservative care for CLBP. Primary exclusion criteria included symptomatic spinal stenosis, disk protrusion > 5 mm, spondylolisthesis > 2 mm at any level, or radiculopathy. Table 1 is a complete listing of inclusion/exclusion criteria for this study. Upon confirmation of eligibility, baseline measurements were collected and entered by the study site research personnel into the online clinical database (iMedNet, Mednet Solutions, Minneapolis, MN).
Study interventions
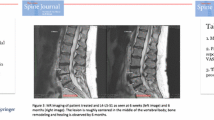
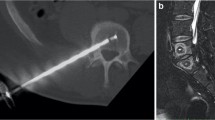
Patients in the study were treated with intraosseous RF ablation of the BVN using a unilateral transpedicular delivery system (Intracept® System, Relievant Medsystems, Minneapolis, MN). The procedure was performed under image guidance, with the patient under moderate conscious sedation or general anesthesia, and in an outpatient setting. Targeted location for electrode placement was approximately 30–50% across vertebral body width from the posterior wall, and in the same horizontal plane as the BVN on sagittal imaging as shown in Fig. 1. After confirmation of placement, thermal ablation was delivered for 15 min at 85 °C to create an approximately 1-cm spherical lesion within each vertebral body as shown in Fig. 2. Detailed information about the surgical technique was described previously in the literature [11]. All patients continued nonsurgical therapies as per the investigator’s medical judgment and patient symptoms.
Outcome measures
The primary endpoint of the study was collected at 3 months posttreatment. Secondary endpoints of clinical outcomes over time are collected at 6, 9, and 12 months. All endpoints were patient self-reported using validated questionnaires. The primary endpoint was a measurement of impact of low back pain on patient function using the Oswestry Disability Index (ODI) questionnaire [15]. Secondary endpoints included measurement of low back pain using a visual analog scale (VAS) [16] with a range of 0 cm (no pain) to 10 cm (worst pain imaginable) and quality of life measurements using the SF-36 [17] and EQ-5D-5L [18, 19] questionnaires.
To confirm targeting success, a 6 weeks post-RF ablation MR image (T1, T2, and STIR time constants) was performed and reviewed by an independent neuroradiologist to assess the degree of overlap of the RF ablation lesion with the terminus of the BVN for each treated vertebral body.
Device or device procedure-related spinal or neurological adverse events (AEs) were collected in this study. All reported AEs were adjudicated by an independent clinical event committee for relatedness to device therapy and procedure.
Statistical analysis
With a block sample of 28 patients, this study was 80% powered at an alpha of 0.05 to a detected 15-point change in ODI from baseline. To evaluate for procedural effectiveness, the patient-reported measures noted above were compared from baseline to 3 months posttreatment. Results were summarized using descriptive statistics. Tests of the changes between the baseline and posttreatment values were performed with Stata v15 (Stata Corp., College Station, TX) using a Student’s two-tailed t test without imputation for missing values.
Study revisions
Upon receiving Food and Drug Administration 510(k) clearance, the protocol was revised to allow treatment of up to four vertebrae and treatment of nonconsecutive levels from L3 to S1. Inclusion of patients at least 6 months from laminectomy or discectomy, with moderate symptomatic spinal stenosis, and taking extended-release opioids, was also allowed. All subjects met the inclusion/exclusion criteria of the final protocol for the analysis. An evaluation of the impact of protocol revisions to the primary endpoint detected no significant differences, and no adjustment for difference was required.
Results
Patient demographics and baseline characteristics
A total of 28 consecutive patients were treated. Median age for this study population was 45 years; baseline ODI was 48.5; VAS was 6.36. The percentage of patients with LBP symptoms ≥ 5 years was 75%. Modic Type 1 was present in 14/28 (50%) of the patients; Type 2 in 42.9%, and both Type 1 and 2 in 7.1% of study patients. Disk protrusion was documented in 24 of the 28 study patients (85.7%), and 7.1% had a diagnosis of spondylolithesis.
Over 61% of patients had previously undergone at least one trial of physical therapy or a formal exercise program; 64% had received chiropractic care; 61% had undergone spinal injections; and 25% were actively using opioids at enrollment. Prior discectomy had been performed in 14.3% of patients. Table 2 provides patient demographics and baseline characteristics.
Treatment results
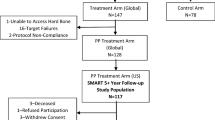
Treatment was attempted in 29 patients, and RF ablation was performed in 28 patients. One patient’s procedure was aborted due to an inability to access the vertebral target area because of unusually hard bone. Two of the RF-treated patients with Modic at L5-S1 received RF ablation at L5 only, due to an inability to access S1. These patients are included as intent-to-treat in the analysis. See Fig. 3 for patient enrollment and follow-up status.
Of the 28 patients treated with RF ablation, 23 (82%) had two vertebrae treated, four patients had three vertebrae treated, and one patient had four vertebrae ablated. The most commonly treated segment was L5-S1 (71%). Targeting was adjudicated as successful in 62/62 (100%) of RF ablation-treated bodies and in 62/64 (97%) of vertebral bodies attempted. No RF ablation patients received a spinal injection posttreatment and prior to the 3-month primary endpoint. Four of the eight patients (50%) using opioids at baseline had discontinued use at 3 months posttreatment. The remaining four patients continued opioid use at the same dosage as baseline (Fig. 4).
Primary endpoint: ODI
The primary endpoint of the study was collected at 3 months posttreatment. Mean change in ODI at 3 months posttreatment was − 30.07 +14.52 points (p < 0.0001) as shown in Fig. 5. Table 3 provides details of the primary endpoint measurements. Using a 10-point improvement threshold for success, 26/28 (92.9%) of patients in the RF ablation arm reached clinical success as shown in Fig. 5. An ODI improvement of ≥ 20 points was reported by 21/28 (75.0%) of RF ablation patients.
Secondary endpoints
VAS and other secondary outcomes are displayed in Table 4. The mean change in VAS was − 3.50 + 2.33 (p < 0.0001). A VAS decrease of 2.0 cm was reported in 21/28 (75.0%) of patients (Fig. 6).
The mean improvement from baseline in overall SF-36 was 26.06 + 15.15 with sub-part results of 15.78 + 9.13 (physical) and 4.23 + 8.84 (mental). Both subparts were statistically significant improvements. Similarly, mean improvement from baseline in EQ-5D-5L was significant with an increase of 0.198 + 0.129 (p < 0.0001).
Nineteen (68%) of patients in this prospective study had 6-month outcomes data for ODI and VAS at the time of reporting these data. These patients continue to report statistically significant improvements from baseline with a mean ODI at 6 months of 13.05 + 11.99 and a mean change in ODI of 36.63 + 15.31 (p < 0.0001). Mean VAS score in these 19 patients at 6 months is 1.42 + 1.77 which is a mean reduction from baseline of 4.95 + 2.12 (p < 0.0001). Similarly, responder rates remain high with 19/19 (100%) of these patients reporting a > 10-point reduction in ODI and 16/19 (84.2%) reporting a > 20-point reduction in ODI.
Patient satisfaction
In the 28 RF ablation-treated patients, 21 (75%) rated their condition as improved, 5 (18%) reported no change, and 2 (7%) indicated worsened condition. Twenty-two patients (78.6%) indicated the treatment was a success and were satisfied with the results of surgery, and 24(85.7%) of the treated patients responded they would have the surgery again for the same condition.
Work status
Eighty-six percent (86%) of subjects were working (79% full-time and 21% part-time) at baseline. Six patients (21%) reported missing work, and 7(25%) patients reported spending > ½ day in bed due to LBP in the 2 weeks prior to baseline. At 3 months posttreatment, patients reported improvements in work function of 71% and 83%, respectively, with only one (1) patient reporting missed work in the prior 2 weeks and two (2) patients spending > ½ day in bed due to LBP.
Adverse events
Adverse events (AEs) that were musculoskeletal, neurological, device-, or procedure-related were collected in this study. Of the 5 AEs reported in 28 RF ablation-treated patients, none were serious or device-related. Three AEs were adjudicated by the clinical event committee as related to the device procedure. One was an aborted procedure due to inability to access, and two were leg pain events with potential pedicle tract issues noted by the clinical event committee in their review of the 6-week MRI. Both were categorized as mild, requiring no interventions beyond oral medication, and were resolved in a mean time of 91 days.
Discussion
After an initial RCT demonstrated the safety and efficacy of BVN RF ablation in the management of CLBP, we sought to understand the procedure’s effectiveness. Patients in this study were treated in a typical, community spine clinic setting by providers with no special expertise in this procedure. Additionally, more broad inclusion and narrow exclusion criteria were employed than in the efficacy study. Patient selection was based on objective MRI findings of Modic changes and clinician judgment; there were no requirements to rule out other sources of pain. This is particularly important in an axial low back pain population, where a variety of “pain generators” are often treated based on diagnostic local anesthetic blocks that rely on a patient’s subjective assessment of pain. In contrast to the efficacy trial, the current study also included patients who had undergone prior discectomy; the maximum number of VB levels treatable was increased from 3 to 4, and the requirement that treatment occurred at consecutive levels was removed. There were no restrictions placed on medications or treatments in follow-up. Lastly, there were no upper limits on ODI or VAS imposed, and the results were based on the ITT analysis that included two patients that received only partial treatments.
In spite of these more permissive requirements, highly significant improvements from baseline for primary endpoint and all secondary endpoints (p < 0.001) were achieved. 93% achieved an ODI reduction of 10 points and 75% a reduction of 20 points.
Four (4) of the 8 patients that had been on opioids at baseline had stopped using them by 3 months. Aside from a short postoperative period, no patients started opioids. Of those 4 patients that had been taking opioids, none increased their dosage. Although the vast majority of patients were working at baseline, absenteeism was reduced and work function improved.
The primary strength of this study is its inclusion of a “real-world” population of chronic low back pain patients in a community-based spine care setting. Yet, the outcomes reported were consistent with those reported in the treatment arm of the efficacy RCT. Potential limitations to generalizability include the use of research coordinators, a medical monitor, and a defined prescreening process. However, pure effectiveness trials are nearly impossible to perform without some research infrastructure to promote population homogeneity and ensure data quality [20, 21]. Additional potential criticisms may include our relatively small sample and short follow-up interval for the primary endpoint. However, durability of the 3-month results up to 24 months has been established previously [12], and we will continue to collect longer term outcomes as a part of this study.
Conclusion
Patients treated with RF ablation of the BVN reported significant improvements from baseline in ODI, VAS, SF-36, and EQ-5D-5L at 3 months and were highly satisfied with their outcomes. This study reaffirms, using a “real-world” environment, the results of a prior efficacy trial on the role of radiofrequency ablation of the BVN for treatment of vertebrogenic CLBP. Using straight-forward, objective MRI findings for patient selection, these data collectively support a favorable risk/benefit profile for RF ablation of the BVN in a population of CLBP patients with few validated treatment alternatives.
References
Shmagel A, Foley R, Ibrahim H (2016) Epidemiology of chronic low back pain in US adults: data from the 2009–2010 National Health and Nutrition Examination Survey. Arthritis Care Res 68:1688–1694
Freburger JK, Holmes GM, Agans R, Jackman AM, Darter JD, Wallace AS et al (2009) The rising prevalence of chronic low back pain. Arch Intern Med 169(3):251–258
Majid K, Truumees E (2007) Incidence and pathophysiology of chronic low back pain. Semin Spine Surg 20(2):87–92
Cheng JS, Lee MJ, Massicotte E, Ashman B, Gruenberg M, Pilcher LE et al (2011) Clinical guidelines and payer policies on fusion for the treatment of chronic low back pain. Spine (Phila Pa 1976) 36(21 Suppl):S144–S163
Dudli S, Fields AJ, Samartzis D, Karppinen J, Lotz JC (2016) Pathobiology of modic changes. Eur Spine J 25(11):3723–3734
Lotz JC, Fields AJ, Liebenberg EC (2013) The role of the vertebral end plate in low back pain Global. Spine J 3(3):153–164
Dudli S, Sing DC, Hu SS, Berven SH, Burch S, Deviren V et al (2017) ISSLS PRIZE IN BASIC SCIENCE 2017: intervertebral disc/bone marrow cross-talk with Modic changes. Eur Spine J 26(5):1362–1373
Antonacci MD, Mody DR, Heggeness MH (1998) Innervation of the human vertebral body: a histologic study. J Spinal Disord 11(6):526–531
Fras C, Kravetz P, Mody DR, Heggeness MH (2003) Substance P-containing nerves within the human vertebral body. An immunohistochemical study of the basivertebral nerve. Spine J 3(1):63–67
Becker S, Hadjipavlou A, Heggeness MH (2017) Ablation of the basivertebral nerve for treatment of back pain: a clinical study. Spine J 17(2):218–223
Fischgrund JS, Rhyne A, Franke J, Sasso R, Kitchel S, Bae H et al (2018) Intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: a prospective randomized double-blind sham-controlled multi-center study. Eur Spine J 27(5):1146–1156
Fischgrund JS, Rhyne A, Franke J, Sasso R, Kitchel S, Bae H, et al (2019) Intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: two-year results from a prospective randomized double-blind sham-controlled multi-center. Study Int J Spine Surg
Rothwell PM (2005) External validity of randomised controlled trials: “to whom do the results of this trial apply?”. Lancet 365:82–93
Singal AG, Higgins PDR, Waljee AK (2014) A primer on effectiveness and efficacy trials Clinical And Translational. Gastroenterology 5:e45
Roland M, Fairbank J (2000) The Roland Morris disability questionnaire and the Oswestry disability questionnaire. Spine 25(24):3115–3124
Price DD, McGrath PA, Rafii A, Buckingham B (1983) The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain 17(1):45–56
Ware JE, Kosinski M, Keller SK (1994) SF-36 physical and mental health summary scales: a user’s manual. The Health Institute, Boston
Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D et al (2011) Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 20(10):1727–1736
Janssen MF, Pickard AS, Golicki D, Gudex C, Niewada M, Scalone L et al (2013) Measurement properties of the EQ-5D-5L compared to the EQ5D-3L across eight patient groups: a multi-country study. Qual Life Res 22(7):1717–1727
Fritz JM, Cleland J (2003) Effectiveness versus efficacy: more than a debate over language. J Orthop Sports Phys Ther 33(4):163–165
Thorpe KE, Zwarenstein M, Oxman AD, Treweek S, Furburg CD, Altman DG et al (2009) A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. J Clin Epidemiol 62(5):464–475
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Financial support for study related costs, paid directly to the institutions, was received from Relievant Medsystems, Minneapolis, MN.
Ethical approval
This research was conducted under the oversight of the Western Institutional Review Board, Advarra IRB, and the investigational site’s local IRB.
Informed consent
Informed consent was obtained for participants in this study. This research was conducted in accordance with the Helsinki Declaration.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Truumees, E., Macadaeg, K., Pena, E. et al. A prospective, open-label, single-arm, multi-center study of intraosseous basivertebral nerve ablation for the treatment of chronic low back pain. Eur Spine J 28, 1594–1602 (2019). https://doi.org/10.1007/s00586-019-05995-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-019-05995-2