Abstract
Patients with chronic non-specific low back pain (LBP) walk with more synchronous (in-phase) horizontal pelvis and thorax rotations than controls. Low thorax–pelvis relative phase in these patients appears to result from in-phase motion of the thorax with the legs, which was hypothesized to affect arm swing. In the present study, gait kinematics were compared between LBP patients with lumbar disc herniation and healthy controls during treadmill walking at different speeds and with different step lengths. Movements of legs, arms, and trunk were recorded. The patients walked with larger pelvis rotations than healthy controls, and with lower relative phase between pelvis and thorax horizontal rotations, specifically when taking large steps. They did so by rotating the thorax more in-phase with the pendular movements of the legs, thereby limiting the amplitudes of spine rotation. In the patients, arm swing was out-of phase with the leg, as in controls. Consequently, the phase relationship between thorax rotations and arm swing was altered in the patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with low back pain (LBP) often report difficulties with walking, and usually walk slower than their healthy peers [1, 2]. Furthermore, gait coordination is changed in these patients. In normal slow walking, horizontal rotations of pelvis and thorax are more or less synchronous (“in-phase”), but at higher speeds, they move more “out-of-phase” (less synchronous). This change of coordination at higher speeds occurs less in patients with chronic non-specific LBP [1, 2]. The same was reported for pregnancy-related pelvic girdle pain (PPP) [3, 4]. It was suggested that patients keep their pelvis and thorax rotations more in-phase to avoid large (or fast) rotations in the spine [4].
In healthy subjects, pelvis rotations are relatively out-of-phase with the pendular movements of the leg at lower walking speeds, but more in-phase at higher speeds [5], while the thorax rotates out-of-phase with the legs at all speeds. This pattern explains the normal shift in thorax–pelvis relative timing from in-phase toward out-of-phase when walking speed increases. For non-specific LBP, the smaller phase differences between thorax and pelvis rotations were suggested to derive from the thorax rotating more in-phase with the legs at higher speeds [4].
Since thorax-rotations are involved in driving the arm swing [6], changes in thorax–leg timing may have consequences for the relationship between arms and legs. If the arms follow the altered timing of the thorax, they will swing more in-phase with the legs, which is energy inefficient [7]. Alternatively, to maintain out-of-phase arm movement relative to the legs, extra shoulder muscle activity may be required. To our knowledge, the impact of adaptations in trunk coordination on the arm swing in walking with LBP has not been studied.
In PPP patients, it was shown that rotations of the pelvis in the transverse plane are larger at higher gait speeds [4], apparently to compensate for hip flexion limitations, which are present in PPP. Until to date, such an increase of pelvis rotation has not been confirmed for LBP patients, in spite of the fact that limitations in hip flexion may also be present in LBP [8–10].
This study is part of a research programme [1–5] that aims at understanding the nature and cause of gait problems in LBP, to identify means to help patients to walk with less difficulty. For the present study, LBP subjects were recruited with a confirmed diagnosis of lumbar disc herniation (LDH). Note, however, that not all LBP patients with LDH have LBP because of the LDH [11–13]. To minimize potential patient discomfort during walking, the study was limited to patients with mild LBP, who were still able to perform their daily activities. In this study, we address three questions: (1) Do LBP patients with LDH walk with larger pelvis rotations? (2) Do these patients adapt the timing of thorax rotations to that of leg movements? (3) In how far does this change in timing affect the timing of arm swing? Since it was recently found that thorax–pelvis relative phase depends on stride length rather than stride frequency [14], not only walking speed, but also stride length was manipulated.
Methods
Low back pain patients with LDH and healthy controls were recruited by word of mouth (both, N = 12). Inclusion criteria were between 20 and 45 years of age, LDH confirmed by CT-scan, pain not beyond the knee, and able to walk a few blocks. Exclusion criteria for both groups were BMI > 30, leg length discrepancy > 2 cm, previous back or leg surgery, or other diseases affecting gait. The local Medical Ethical Committee approved the protocol, and subjects provided informed consent.
Procedure
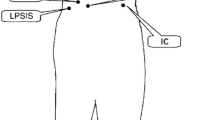
Experimental methods were similar to previous studies [2, 5]. Patients rated their current pain intensity on a Visual Analogue Scale (VAS), from “no pain” (0 mm) to “maximal pain” (100 mm). For movement registration, neoprene bands with clusters of three infrared light emitting diodes (LEDs) were attached at the thorax (T6), the lumbar segment (L3), pelvis (level of the posterior superior iliac spines), left forearm, thigh, and calcaneus (Fig. 1). LED movements were recorded with a 2 × 3 camera array (OPTOTRAK Certus, Northern Digital Inc., Waterloo, ON, USA). The position of the ulnar styloid relative to the forearm cluster was determined in a separate measurement using a pointer with six LEDs. Reference measurements in the anatomical position allowed for aligning the coordinate system of each cluster marker with the global coordinate system (x-axis forward, y-axis to the left, and z-axis upward).
During the experiment, subjects walked on a treadmill (EN-BO system, Bonte technology, Amsterdam, The Netherlands) at increasing speeds (1.0, 2.5, 4.0, and 5.5 km/h), with normal steps, small steps, and large steps, respectively. In each condition, 20 s of measurement (50 samples/s) started after 15 s of warming-up. Participants were asked to indicate when walking speed was too high, at which point the experiment would be stopped.
Basic gait parameters
Heel strikes were determined from the vertical minima of the heel marker, stride time as the time between consecutive heel strikes, stride frequency as 1 divided by stride time, and stride length as stride time multiplied by treadmill speed.
Amplitudes
Time series of trunk and pelvis segmental rotations around the z-axis were calculated. In addition, time series of leg movement were derived from the x-positions of the thigh marker. Time series of arm swing were constructed from the x-position of the ulnar styloid, estimated from cluster marker locations [15]. All time series were filtered with a fourth order bi-directional Butterworth filter, cut-off frequency 5 Hz.
Amplitudes were calculated as the absolute difference between maximum and minimum within one stride cycle, averaged per condition. Spine rotations were obtained by subtracting the relevant time series from each other: Lumbar spine rotation as lumbar segment minus pelvis, thoracic spine as thorax minus lumbar segment, and total spine as thorax minus pelvis.
Relative Fourier Phase
Fourier phase of the trunk rotations and arm and leg swing was calculated [1] to express phase (=timing) at the fundamental frequency of the leg. Subsequently, Relative Fourier Phase (RFP) time series were obtained by subtracting the phase of the cranial segments from those of the caudal segments. Mean RFP was calculated per condition by using circular statistics [16]. When the left side of the pelvis and the upper leg were both in their most forward position at the same time, their RFP would be expressed as 0° (“in-phase”), and when one was most forward, with the other most backward, as 180° (“anti-phase”). All calculations were performed with MATLAB 7.4 (the Mathworks, Natick, MA, USA).
Statistical analysis
Subject characteristics were compared between groups using unpaired t tests. Generalized Estimation Equations (GEEs) were used (SPSS 16.0) for all variables to analyze the effects of Group (patients vs. controls), Speed (4 levels), Step (small/normal/large), and their interactions. Non-significant interactions were removed stepwise. P < 0.05 was considered significant.
Results
No significant differences between controls and patients were found in age (37.8 ± 4.2 vs. 37.4 ± 4.2 years, respectively), height (164.9 ± 9.1 vs. 165.2 ± 9.7 cm), weight (64 ± 14.7 vs. 62.5 ± 14.9 kg), or BMI (23.3 ± 3.6 vs. 22.6 vs. 3.5). In the patients (Table 1), the VAS-score for pain was 39 ± 19 mm, significantly different from 0 (one-sample t test, P = 0.00). Patients’ symptoms had lasted between 2 weeks and 15 years. CT-scans revealed central herniations at L3–L4–L5–S1.
Basic gait parameters
All participants could walk at all speeds. In all conditions, patients walked with similar stride length and frequency as controls (Tables 2, 3). When subjects were asked to walk with bigger/smaller steps than normal, at the same speed, they clearly did so (P values 0.00). Stride length and frequency increased with increasing walking speed (P values 0.00).
Rotational amplitudes
Pelvis rotational amplitude (Fig. 2) was significantly affected by Group, with patients having larger amplitudes (P = 0.04). Moreover, pelvis rotational amplitudes increased with step length (P = 0.00) and speed (P = 0.00), which was more pronounced for larger steps (Step × Speed, P = 0.04).
Thorax rotational amplitude increased with increasing step length (P = 0.00), but decreased with increasing speed, more so with larger steps (Step × Speed, P = 0.00). Lumbar rotational amplitude increased with increasing step length (P = 0.00), without significant effect of speed. Increasing step length increased lumbar spine rotational amplitude (P = 0.00), and all spinal rotations increased with increasing walking speed (P = 0.00), for thoracic spine and total spine rotation more so with large steps (Step × Speed, P = 0.00).
Relative Fourier Phase
When walking with larger steps, patients’ thorax–pelvis and thorax–lumbar RFP (Fig. 3) were lower than in controls (Group × Step, P values 0.01). Moreover, at higher speeds, the patients walked with lower thorax–leg relative phase (Group × Speed, P = 0.02).
Increasing step length coincided with more out-of-phase movements of the thorax and the lumbar segment, but more in-phase movements between thorax and leg (P values 0.00). The relative phase between the lumbar segment and the leg, and between the pelvis and the leg decreased with increasing step length (P values 0.00). With increasing speed, the thorax moved more out-of-phase with the other segments, and the pelvis more out-of-phase with the lumbar segment, while the pelvis and lumbar segment moved more in-phase with the leg (P values ≤ 0.01). All these effects, except for lumbar-pelvis RFP, were dependent upon step length (Step × Speed, P = 0.00).
Arm swing
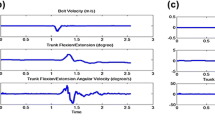
Arm swing amplitude (Fig. 4) was affected by Step (P = 0.01), with larger values for normal steps. Arm swing also increased with Speed (P = 0.00), particularly when walking with normal steps (Step × Speed, P = 0.00).
At higher speeds, patients swung their arm more out-of-phase with the thorax than controls (Group × Speed, P = 0.03), while arm–leg RFP revealed no effect of, or interaction with, Group. With larger steps, thorax–arm relative phase increased (P = 0.00), as with increasing speed (P = 0.00), particularly when normal steps were used (Step × Speed, P = 0.01). With large steps, arm–leg relative phase was lower at lower speed (P = 0.00), and increased with increasing speed (Step × Speed, P = 0.00).
Discussion
During treadmill walking, LBP patients with lumbar disc herniation (LDH) had larger pelvis rotations than controls. Moreover, at higher speeds/with larger steps, patients’ thorax rotations were less out-of-phase (more synchronous) with lumbar and pelvis rotations, and with the pendular movements of the legs. Arm swing kept its normal out-of-phase relation with the legs, and was therefore more out-of-phase with respect to the thorax.
Pelvis rotations
In non-specific LBP, no change in the amplitude of pelvis rotations during gait was reported, but variability is high [1, 2, 17]. Increased pelvis rotations were found in pregnancy-related pelvic girdle pain (PPP) [3, 4], as in the present study of LBP with LDH. The Straight Leg Raise is limited in LDH, probably because hip flexion is limited [18], as is the case in PPP [19].
One LDH study [20] reported an active range of hip flexion of just 42° ± 19°. Since hip flexion is important to increase stride length, one could expect LDH patients to walk with smaller steps. This would, however, be less energy efficient [21], and in the present study, LBP with LDH did not affect stride length. At higher speeds, pelvis rotations contribute to step length [14, 22]. Thus, the most plausible explanation of the larger pelvis rotations in the present study is that patients used more pelvis rotation to maintain stride length, thereby compensating for limited hip flexion.
Trunk coordination
In non-specific LBP [1, 2], and PPP [3, 4], thorax–pelvis Relative Fourier Phase (RFP) increases less with increasing speed than in controls. In the present study, similar, but more subtle results were found, i.e., thorax–pelvis (and thorax–lumbar) RFP was only reduced when patients walked faster with large steps. Rather than assuming that LBP with LDH is less serious than non-specific LBP or PPP, the present study may have given less pronounced results because patients were only mildly affected (with an average pain-score of 39 mm, and able to walk at all speeds).
The larger pelvis rotations in the patients would be expected to lead to large spine rotations, which could be painful in LDH. However, patients changed the timing of their thorax movements, bringing the thorax more in-phase with the legs, and thereby reduced thorax–pelvis RFP. In this way, patients could keep their spine rotations in check [4], also when walking fast with large steps. Post hoc inspection of the individual data suggested that patients with the largest pelvis rotations had the smallest thorax–leg RFP.
Arm swing
Thorax rotation plays a role in driving arm swing [6], and with increased synchrony between thorax and legs, more synchrony of arm and leg movements might be expected. However, the amplitude of arm swing, and arm–leg RFP, was unaffected, while arm–thorax RFP was increased in the patients. Clearly, the arms were kept out-of-phase with the leg—probably because this is energetically more efficient [7].
To the best of our knowledge, altered relative timing of the arm swing with respect to thorax rotations has not been reported previously. This result may illustrate that the control system deals with conflicting constraints [23], such as optimizing energy, avoiding pain, and maintaining stability. Each form of pathology requires adaptations, and the present study suggests an adaptation (increased arm–thorax RFP) to an adaptation (decreased thorax–leg RFP) to an adaptation (increased pelvis rotations) to an adaptation (decreased hip flexion) to pain (LDH).
Clinical relevance
Treatment and diagnosis of LBP with LDH are beyond the scope of the present study. However, several results may be clinically relevant: (1) increased pelvis rotations during walking (as can be seen with the naked eye) appear to point at limitations in hip flexion; (2) trunk coordination during walking in LBP patients with LDH suggests that they attempt to prevent large or fast spine rotations; (3) to keep arm swing out-of-phase with the legs, the trunk adaptations in LBP with LDH may lead to increased muscle activity in the shoulder, and simultaneous prevalence of low back and shoulder pain may thus be related.
Study limitations
To minimize patient discomfort, we offered conditions in one standardized sequence, which may have introduced some order effects, but results largely agreed, or were consistent, with earlier findings [2, 4, 5, 14]. Subjects were Chinese, and there may be ethnic [24] and cultural [25] effects on relevant parameters (e.g., smaller “normal” steps). Moreover, some of the results were subtle, but patients had mild complaints only. Finally, it was concluded that hip flexion was limited, but this was not measured, and the results appeared to imply a change in shoulder muscle activity in walking with LDH, but shoulder muscle activity was not recorded.
Conclusion
LBP patients with LDH walked with larger pelvis rotations than healthy controls, and reduced relative phase between pelvis and thorax horizontal rotations, specifically when taking large steps. They did so by rotating the thorax more in-phase with the pendular movements of the legs, which allowed them to limit amplitudes of spine rotations. In the patients, arm swing was out-of-phase with the leg, as in controls. Consequently, the phase relationship between thorax rotations and arm swing was altered in patients.
References
Lamoth CJC, Meijer OG, Wuisman PILM, Van Dieën JH, Levin MF, Beek PJ (2002) Pelvis–thorax coordination in the transverse plane during walking in persons with nonspecific low back pain. Spine 15:E92–E99
Lamoth CJC, Daffertshofer A, Meijer OG, Beek PJ (2006) How do persons with chronic low back pain speed up and slow down? Trunk–pelvis coordination and lumbar erector spinae activity during gait. Gait Posture 23:230–239
Wu WH, Meijer OG, Jutte PC, Uegaki K, Lamoth CJC, De Wolf GS, Van Dieën JH, Wuisman PIJM, Kwakkel G, De Vries JIP, Beek PJ (2002) Gait in patients with pregnancy-related pain in the pelvis: an emphasis on the coordination of transverse pelvic and thoracic rotations. Clin Biomech 17:678–686
Wu WH, Meijer OG, Bruijn SM, Van Dieën JH, Lamoth CJC, Van Royen BJ, Beek PJ (2008) Gait in pregnancy-related pelvic girdle pain: amplitudes, timing, and coordination of horizontal trunk rotations. Eur Spine J 17:1160–1169
Bruijn SM, Meijer OG, Van Dieën JH, Kingma I, Lamoth CJC (2008) Coordination of leg swing, thorax rotations, and pelvis rotations during gait: the organisation of total body angular momentum. Gait Posture 27:455–462
Pontzer H, Holloway JH 4th, Raichlen DA, Lieberman DE (2009) Control and function of arm swing in human walking and running. J Exp Biol 212:523–534
Collins SH, Adamczyk KG, Kuo AD (2009) Dynamic arm swinging in human walking. Proc Biol Sci 276:3679–3688
Blankenbaker DG, Haughton VM, Rogers BP, Meyerand ME, Fine JP (2006) Axial rotation of the lumbar spinal motion segments correlated with concordant pain on discography: a preliminary study. Am J Roentgenol 186:795–799
Veres SOP, Robertson PA, Broom ND (2009) The morphology of acute disc herniation: a clinically relevant model defining the role of flexion. Spine 34:2288–2296
Wong TK, Lee RY (2004) Effects of low back pain on the relationship between the movements of the lumbar spine and hip. Hum Mov Sci 23:21–34
Powell MC, Wilson M, Szypryt P, Symonds EM, Worthington BS (1986) Prevalence of lumbar disc degeneration observed by magnetic resonance in symptomless women. Lancet 2:1366–1367
Jensen MC, Brant-Zawadzki MN, Obuchowski N, Modic MT, Malkasian D, Ross JS (1994) Magnetic Resonance Imaging of the lumbar spine in people without back pain. New Engl J Med 331:69–73
Keller RB, Atlas SJ, Sould DN, Singer DE, Deyo RA (1998) Lumbar disc herniation and spinal stenosis. J Bone Joint Surg Am 81:752–762
Huang YP, Meijer OG, Lin JH, Bruijn SM, Wu WH, Lin XC, Hu H, Huang CH, Shi L, Van Dieën JH (2010) The effects of stride length and stride frequency on trunk coordination in human walking. Gait Posture 31:444–449
Kingma I, De Looze MP, Toussaint HM, Klijnsma HG, Bruijnen TB (1996) Validation of a full body 3-D linked segmental model. Hum Mov Sci 15:833–860
Fisher NI (1993) Statistical analysis of circular data. Cambridge University Press, Cambridge
Vogt L, Pfeifer K, Portscher K, Banzer W (2001) Influences of nonspecific low back pain on three-dimensional lumbar spine kinematics in locomotion. Spine 26:1910–1919
Majlesi J, Togay H, Unalan H, Toprak S (2008) The sensitivity and specificity of the Slum and the Straight Leg Raising tests in patients with lumbar disc herniation. J Clin Rheumatol 14:87–91
Hu H, Meijer OG, Van Dieën JH, Hodges PW, Bruijn SM, Strijers RL, Nanayakkara PW, Van Royen BJ, Wu W, Xia C (2009) Muscle activity during the active straight leg raise (ASLR), and the effects of a pelvic belt on the ASLR and on treadmill walking. J Biomech 43:532–539
Mannion AF, Dvorak J, Müntener M, Grob D (2008) A prospective study of the interrelationship between subjective and objective measures of disability before and 2 months after lumbar decompression surgery for disc herniation. Eur Spine J 14:454–465
Bertram JE, Ruina A (2001) Multiple walking speed-frequency relations are predicted by constrained optimization. J Theor Biol 209:445–453
Ducroquet R, Ducroquet J, Ducroquet P (1965) Walking and limping: a study of normal and pathological walking patterns. Lippincott, Philadelphia
Van Dieën JH (2007) Low back pain and motor behavior: contingent adaptations, a common goal. In: Diagnosis and treatment, the balance between research and clinic. Proceedings of the 6th interdisciplinary world congress on low back pain & pelvic pain. Barcelona, November 7–10, pp 3–14
Ryu T, Choi HS, Choi HW, Chung MK (2006) A comparison of gait characteristics between Korean and Western people for establishing Korean gait reference data. Int J Ind Ergon 36:1023–1030
Rush REC, Freitas I, Plank LD (2009) Body size, body composition and fat distribution: comparative analysis of European, Maori, Pacific Island and Asian Indian adults. Br J Nutr 102:632–641
Acknowledgments
The study was performed at the Orthopaedic Biomechanics Lab of the Second Affiliated Hospital of Fujian Medical University. The authors thank their hosts, Profs. Lin Ling and Lv Gorong, Second Affiliated Hospital of FMU, Quanzhou, Fujian, People’s Republic of China, as well as Li Weiping, Deputy General Secretary of FMU, Fuzhou, Fujian, People’s Republic of China, for their stimulating enthusiasm. The study was supported by grant 2008-Z39 from the Science and Research Bureau (YPH), Quanzhou, Fujian, People’s Republic of China; by grant NCETFJ-0611 from the Programme for New Century Excellent Talents of Fujian University (WHW, XCL. OGM), Fuzhou, Fujian, People’s Republic of China; and by grants from Biomet Nederland, Dordrecht, The Netherlands (SMB, HAB). The Quanzhou–Amsterdam cooperation was stimulated by Biomet Nederland and Biomet Europe. The authors thank Hans van den Berg and Tijmen van Dam for their inspiration.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Huang, Y.P., Bruijn, S.M., Lin, J.H. et al. Gait adaptations in low back pain patients with lumbar disc herniation: trunk coordination and arm swing. Eur Spine J 20, 491–499 (2011). https://doi.org/10.1007/s00586-010-1639-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-010-1639-8