Abstract
Previous papers on resorbable poly-l-lactide-co-d,l-lactide (PLDLLA) cages in spinal fusion have failed to report adequately on patient-centred clinical outcome measures. Also comparison of PLDLLA cage with a traditionally applicable counterpart has not been previously reported. This is the first randomized prospective study that assesses clinical outcome of PLDLLA cage compared with a poly-ether-ether-ketone (PEEK) implant. Twenty-six patients were randomly assigned to undergo instrumented posterior lumbar interbody fusion (PLIF) whereby either a PEEK cage or a PLDLLA cage was implanted. Clinical outcome based on visual analogue scale scores for leg pain and back pain, as well as Oswestry Disability Index (ODI) and SF-36 questionnaires were documented and analysed. When compared with preoperative values, all clinical parameters have significantly improved in the PEEK group at 2 years after surgery with the exception of SF-36 general health, SF-36 mental health and SF-36 role emotional scores. No clinical parameter showed significant improvement at 2 years after surgery compared with preoperative values in the PLDLLA patient group. Only six patients (50%) in the PLDLLA group showed improvement in the VAS scores for leg and back pain as well as the ODI, as opposed to 10 patients (71%) in the PEEK group. One-third of the patients in the PLDLLA group actually reported worsening of their pain scores and ODI. Three cases of mild to moderate osteolysis were seen in the PLDLLA group. Following up on our preliminary report, these 2-year results confirm the superiority of the PEEK implant to the resorbable PLDLLA implant in aiding spinal fusion and alleviating symptoms following PLIF in patients with degenerative spondylolisthesis associated with either canal stenosis or foramen stenosis or both and emanating from a single lumbar segment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Technical evolution in posterior lumbar interbody fusion (PLIF) devices has witnessed the application of cage implants fabricated from various metallic and non metallic materials with a varying range of geometric design. Cages fabricated from poly-ether-ether-ketone (PEEK), when impacted with autologous bone graft, have been shown to promote spinal interbody fusion with high fusion rates and good to excellent clinical outcome reported in the literature [6, 11–13]. More recently a resorbable implant fabricated from poly-l-lactide-co-d,l-lactide (PLDLLA) was introduced into clinical practice as a structural support and bone graft containment device intended to aid lumbar spinal interbody fusion [2, 4, 10, 16]. Although early papers were overwhelmingly promising, appraisal of the current literature shows that little subsequent clinical data on resorbable cages have emerged and clinical application of these implants seems to be diminishing. Whereas several authors have reported fusion rates as high as 92–100% after PLIF with the aid of a PLDLLA cage [2–5, 8–10], virtually none of these studies have prospectively assessed patient-centred clinical outcome measures in their entire study population. Also patients were not randomized to compare with existing treatment options. The objective of this study was to compare the clinical outcome of lumbar interbody fusion using Telamon PEEK™ with that of Telamon PLDLLA Hydrosorb™ (Both Medtronic Sofamor Danek, Memphis, TN, USA) fusion devices in a prospective randomized clinical trial, at a minimum of 2-year follow-up.
Materials and methods
Twenty-six patients with chronic back pain and irradiating lower extremity symptoms were randomly assigned to undergo instrumented posterior lumbar interbody fusion whereby either a non resorbable Telamon PEEK cage or a resorbable Telamon PLDLLA cage was implanted to aid fusion. Randomisation was performed by means of block stratification over time. Stratification of secondary patient characteristics in the block was not performed. All patients had degenerative spondylolisthesis and in addition either a canal stenosis, foramen stenosis or both. Also all patients had lower back as well as irradiating lower extremity symptoms. The diagnosis was supported by pre-operative radiographs (antero-posterior and lateral views as well as flexion and extension views) and MRI-scans in all patients. The study protocol was approved by the institutional review board and ethical committee, whilst an informed consent was obtained from each patient. After surgery unrestricted ambulation was permitted without any external supportive device. Upon discharge, follow-up visits were at 3, 6, 12 and 24 months after surgery. A 10-point VAS score, ODI and SF-36 questionnaire were obtained preoperatively and at each follow-up visit. A VAS score of zero indicates no pain whilst a VAS score of 10 indicates the worst imaginable pain. For the SF-36 questionnaires a physical component score (PCS), mental component score (MCS) and eight summary scores (physical functioning, role physical, bodily pain, mental health, social function, vitality, role emotional, and general health) were calculated for each patient. Scores were normalized to the general Dutch population so that a score of 50 represents the score of the general population [1]. Fusion rate was assessed on CT-scans at 24 months after surgery. Preoperative, intra-operative, and follow-up examination data were entered into a study-specific database. All statistical analyses were performed with SPSS 15.0 for Windows. Repeated measures analyses of variance were performed on all dependent variables to test for differences between pre-operative values and post-operative values. In addition, correlation between outcome variables was assessed using the Pearson correlation (r) for continuous, and the point-biserial correlation coefficient (r pb) for correlation between dichotomous and continuous variables. For categorical and ordinal variables a Chi-square analysis was performed, and a Fisher’s exact two-sided p value was computed. To test for difference in performance between cages, the relative post-operative scores (difference between pre- and post-operative values) were compared using ANOVA. A p value <0.05 was considered statistically significant.
Results
PEEK cages were implanted in 14 patients and resorbable PLDLLA cages were implanted in 12 patients. One patient (PEEK cage) was lost to follow-up at 1 year after surgery but was subsequently traced for a 2-year post surgery assessment. Table 1 shows the baseline characteristics of both patient groups. Considering the whole study population, the average VAS score for back pain and leg pain, respectively, improved from 6.1 [standard error of mean (SEM) 0.4] prior to surgery to 4.0 (SEM 0.6, p = 0.012) and from 5.0 (SEM 0.5) to 2.7 (SEM 0.6, p = 0.07) at 2 years after surgery. The average preoperative ODI of 20.5 (SEM 1.3) improved to 13.9 (SEM 2.2, p = 0.014) at 2 years after surgery, whilst average values for the SF-36 physical component score improved from 30.4 (SEM 1.8) prior to surgery, to 38.8 (SEM 2.9, p = 0.004) at 2 years of follow-up. Clinical outcome variables were interrelated. The VAS, ODI and SF-36 PCS all showed significant correlations varying from r = 0.42 (VAS legs and VAS back, p = 0.029) to r = 0.83 (SF-36 PCS and ODI, p = 0.000). When compared with preoperative values, all clinical parameters significantly improved in the PEEK group at 2 years after surgery compared with preoperative values with the exception of SF-36 general health, SF-36 mental health and SF-36 role emotional scores (Table 2). No single clinical parameter showed significant improvement at 2 years after surgery compared with preoperative values in the PLDLLA patient group. Only six patients (50%) in the PLDLLA group showed more than 10% improvement in the VAS cores for leg and back pain as well as the ODI, as opposed to 10 patients (71%) in the PEEK group. One-third of the patients in the PLDLLA group actually reported more than 10% worsening of their pain scores and ODI. Figure 1 shows the degree of decline in pain scores for each treatment group at various time intervals of follow-up. Both treatment groups demonstrate a greater improvement at 6–12 months which is not sustained at 2 years of follow-up. Based on CT scan assessment, 13 out of 14 patients demonstrated solid fusion in the PEEK group (92% fusion rate) whereas 6 out of 12 patients in the PLDLLA group (50% fusion rate) showed solid fusion. This difference in fusion rate is statistically significant p = 0.026. Fusion rate was significantly related to VAS legs (r pb = 0.49, p = 0.01), VAS back (r pb = 0.40, p = 0.04) and SF-36 PCS (r pb = 0.40, p = 0.04). There was a significantly higher rate of subsidence in the PLDLLA group compared with the PEEK group (p = 0.0414).
Three patients (all with non fusion) demonstrated signs of osteolysis in the PLDLLA treatment group. Two patients out of the six radiological failures (PLDLLA group) reported in the preliminary paper have been revised. One case with symptomatic non fusion and screw breakage in S1 underwent an anterior interbody fusion using a PEEK cage whilst a postero-lateral fusion was performed in a second patient. Both patients reported worsening of their pain scores and disability prior to revision surgery.
Discussion
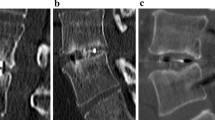
Despite the small patient population, this study demonstrates that PLIF significantly improves pain (VAS scores) and disability (ODI) (Fig. 2) symptoms in patients with symptomatic single segment degenerative spondylolisthesis. PLIF with the aid of PEEK cages have been more extensively studied, with clinical outcome as high as 86% improvement based on patient-centred outcome measures [6, 11]. Our result of 71% improvement in the PEEK group is in concordance with the findings in the literature. Most published data on resorbable cages to date, however, have focused on relatively short-term surgical outcome rather than patient-centred outcome measures of pain, disability and capacity for work. Coe and Vaccarro published a series of 31 patients who underwent PLIF with the aid of PLDLLA implants in which only 16 patients were assessed using SF-36 questionnaires [4]. They reported statistically significant improvement in the 12- and 24-month mean pain scores and 24-month mean role physical scores compared with the preoperative scores on the same scales. These findings contrast with our current results in the PLDLLA patient group. At a minimum of 2-year follow-up, only 50% of the patients in the PLDLLA group demonstrated improvement in the VAS scores for leg and back pain as well as the ODI, as opposed to 71% in the PEEK patient group. The poorer clinical outcome (based on VAS scores, ODI and SF-36) with the PLDLLA implant is consistent with the poorer fusion rate reported in the PLDLLA patient group, and casts some serious doubts on the efficacy of PLDLLA cage in relieving symptoms and enhancing posterior lumbar spinal interbody fusion. We belive that the low fusion rate observed with PLDLLA cages is a result of early mechanical failure leading to loss of structural support prior to the establishment of a fusion mass. Preclinical in vivo studies have demonstrated this fact, with loss of mechanical integrity of PLDLLA cage implants as early as 3 months after implantation, a feature not observed in a PLLA cage another resorbable counterpart [14, 15]. This preclinical finding suggests that the composition of the two PLA isomers in the PLDLLA cage may confer inherent time-dependent mechanical weakness as compared with the mono isomer PLLA cage. Perhaps an even more important factor that could have lead to early mechanical failure and non fusion in the PLDLLA cage is the method of sterilization. All PLDLLA cages used in this study were sterilized by E-beam irradiation. Preclinical in vitro studies have demonstrated the adverse effects of irradiation sterilization techniques on the time-dependent mechanical properties of PLA-based polymers [14]. Ethylene oxide sterilization technique has been shown to be less detrimental in this respect. Therefore, E-beam irradiation may have contributed to a preliminary breakdown of the cage and consequently the higher rate of non union in our study (Fig. 3).
Worsening of symptoms seen in 33% of the patients in the PLDLLA group remain worrisome, and may be a result of the lower fusion rate proffered by the resorbable cage. This is supported by significant correlations between poor fusion rates on the one hand and worsening of pain scores on the other hand. The occurrence of osteolysis and a relatively high rate of subsidence associated with the PLDLLA cage may also be important contributing factors in the poorer clinical outcome as observed in this patient group. The higher rate of subsidence is most likely related to early mechanical failure which in itself can be an important contributory factor in the occurrence of osteolysis. It is, however, important to note that a potential underlying low-grade infection has not been ruled out in these cases of osteolysis. In both treatment groups, initial improvement at 6–12 months after surgery is apparently not sustained at 2 years of follow-up. This finding is consistent with other reports in the literature [7]. We have found no underlying reason for worsening of symptoms at 2 years in our study population.
Following up on our preliminary report, the 2-year results confirm the superiority of the PEEK implant to the resorbable PLDLLA implant in aiding spinal fusion and alleviating symptoms following PLIF in patients with degenerative spondylolisthesis associated with either canal stenosis or foramen stenosis or both and emanating from a single lumbar segment.
References
Aaronson NK, Muller M, Cohen PD, Essink-Bot ML, Fekkes M, Sanderman R et al (1998) Translation, validation, and norming of the Dutch language version of the SF-36 Health Survey in community and chronic disease populations. J Clin Epidemiol 51:1055–1068
Austin RC, Branch CL Jr, Alexander JT (2003) Novel bioabsorbable interbody fusion spacer-assisted fusion for correction of spinal deformity. Neurosurg Focus 14:e11
Coe JD (2004) Instrumented transforaminal lumbar interbody fusion with bioabsorbable polymer implants and iliac crest autograft. Neurosurg Focus 16:E11
Coe JD, Vaccaro AR (2005) Instrumented transforaminal lumbar interbody fusion with bioresorbable polymer implants and iliac crest autograft. Spine 30:S76–S83
Couture DE, Branch CL Jr (2004) Posterior lumbar interbody fusion with bioabsorbable spacers and local autograft in a series of 27 patients. Neurosurg Focus 16:E8
Cutler AR, Siddiqui S, Mohan AL, Hillard VH, Cerabona F, Das K (2006) Comparison of polyetheretherketone cages with femoral cortical bone allograft as a single-piece interbody spacer in transforaminal lumbar interbody fusion. J Neurosurg Spine 5:534–539
Ekman P, Moller H, Hedlund R (2005) The long-term effect of posterolateral fusion in adult isthmic spondylolisthesis: a randomized controlled study. Spine J 5:36–44
Kuklo TR, Rosner MK, Polly DW Jr (2004) Computerized tomography evaluation of a resorbable implant after transforaminal lumbar interbody fusion. Neurosurg Focus 16:E10
Lanman TH, Hopkins TJ (2004) Lumbar interbody fusion after treatment with recombinant human bone morphogenetic protein-2 added to poly(l-lactide-co-d,l-lactide) bioresorbable implants. Neurosurg Focus 16:E9
Lowe TG, Coe JD (2002) Bioresorbable polymer implants in the unilateral transforaminal lumbar interbody fusion procedure. Orthopedics 25:s1179–s1183
Rousseau MA, Lazennec JY, Saillant G (2007) Circumferential arthrodesis using PEEK cages at the lumbar spine. J Spinal Disord Tech 20:278–281
Sears W (2005) Posterior lumbar interbody fusion for lytic spondylolisthesis: restoration of sagittal balance using insert-and-rotate interbody spacers. Spine J 5:161–169
Sears W (2005) Posterior lumbar interbody fusion for degenerative spondylolisthesis: restoration of sagittal balance using insert-and-rotate interbody spacers. Spine J 5:170–179
Smit TH, Engels TA, Wuisman PI, Govaert LE (2008) Time-dependent mechanical strength of 70/30 poly(l, dl-lactide): shedding light on the premature failure of degradable spinal cages. Spine 33:14–18
Smit TH, Krijnen MR, van Dijk M, Wuisman PI (2006) Application of polylactides in spinal cages: studies in a goat model. J Mater Sci Mater Med 17:1237–1244
Vaccaro AR, Singh K, Haid R, Kitchel S, Wuisman P, Taylor W et al (2003) The use of bioabsorbable implants in the spine. Spine J 3:227–237
Acknowledgments
The authors acknowledge with profound gratitude the immense contributions of Paul Wuisman MD, PhD to the study, who unfortunately passed away prior to the preparation of this manuscript. This study was financially supported by Medtronic Sofamor Danek, Memphis, TN, USA.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Jiya, T.U., Smit, T., van Royen, B.J. et al. Posterior lumbar interbody fusion using non resorbable poly-ether-ether-ketone versus resorbable poly-l-lactide-co-d,l-lactide fusion devices. Clinical outcome at a minimum of 2-year follow-up. Eur Spine J 20, 618–622 (2011). https://doi.org/10.1007/s00586-010-1568-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-010-1568-6