Abstract
A 7-year-old Japanese Black cow with anorexia was presented at a local veterinarian. As rectal palpation revealed an enlarged induration of the uterus, lymphoma was suspected, and the patient was introduced to the Veterinary Teaching Hospital of Obihiro University of Agriculture and Veterinary Medicine. Although lymphadenopathy and lymphocytosis were not found, a high proviral load of bovine leukemia virus (BLV) with 464 copies/10 ng DNA was recorded, and lymphocytes with constricted or flower-like nuclei were found occasionally in the peripheral blood; accordingly, enzootic bovine leukosis (EBL) was suspected. According to PCR for clonality analysis of B cells based on immunoglobulin heavy chain (IGH) gene rearrangement, monoclonal proliferation of peripheral blood B cells was evident, suggesting the presence of tumorigenic B cells in the peripheral blood and onset of EBL. Pathological examination including necropsy and histopathological examination confirmed the diagnosis of EBL. Clonality analysis for B cells in the peripheral blood is thought to be useful for diagnosing B-cell tumors such as EBL, especially in cases lacking lymphadenopathy and lymphocytosis. This is the first clinical case of EBL diagnosed by demonstrating monoclonal proliferation of peripheral blood B cells using IGH gene rearrangement-based PCR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Enzootic bovine leukosis (EBL) is caused by bovine leukemia virus (BLV) infection, but infection does not always lead to lymphoma onset (Angelos and Thurmond 2015; Constable et al. 2017). Typically, lymphadenopathy and lymphocytosis are found in patients with EBL, and tumor cells proliferate into multiple organs and lymph nodes throughout the body, occasionally forming masses that press on surrounding organs, resulting in various clinical symptoms (Angelos and Thurmond 2015; Constable et al. 2017). Thus, EBL is clinically suspected in cases where lymphadenopathy is evident on the surface or in the pelvic cavity, and lymphocytosis with atypical lymphocytes is found in the peripheral blood. Enlarged lymph node cytology by fine needle aspiration is usually used for diagnosis of lymphoma (Angelos and Thurmond 2015). However, it is difficult to suspect or diagnose EBL before necropsy in cases lacking lymphadenopathy and lymphocytosis. It is also difficult to detect and distinguish between ‘abnormal lymphocytes’ defined as neoplastic lymphocytes and non-neoplastic cells such as reactive lymphocytes in the peripheral blood (Harvey 2012). The present report is of a clinical case of EBL in a Japanese Black cow lacking both lymphadenopathy and lymphocytosis by demonstrating monoclonal neoplastic proliferation of B cells in the peripheral blood using PCR for clonal analysis based on immunoglobulin heavy chain gene (IGH) rearrangement.
Case presentation
The case was a 7-year-0-month-old Japanese Black cow with anorexia who had delivered a calf 3 months prior to presentation at a local veterinarian. Physical examination on the first day (day 1) showed no abnormalities in the patient’s general condition. Although no superficial lymphadenopathy was observed, rectal examination revealed an enlarged induration of the uterus. No internal iliac lymphadenopathy was recorded. Cytology of the uterus could not be performed. Given that the patient had positive BLV antibodies according to examination performed by the veterinary service office of the local government, and since a case of EBL had been reported at the same farm within the past 2 years, EBL was suspected by the local veterinarian. The case was admitted to the Veterinary Teaching Hospital of Obihiro University of Agriculture and Veterinary Medicine for additional examination for EBL diagnosis on day 5.
On arrival at the hospital, body temperature was 38.5 °C, heart rate was 84 beats/min, and respiratory rate was 18 breaths/min. Activity and body condition of the patient indicated no problems (Fig. 1). Rectal examination revealed an enlarged induration of the uterus from the cervical canal to the uterine body, with no enlargement of the internal iliac lymph nodes. Complete blood count showed no lymphocytosis (Table 1). Giemsa staining of a peripheral blood smear showed that approximately 10% of lymphocytes were atypical with constricted nuclei or flower-like nuclei in morphology; nuclei were medium in size with a diameter of two erythrocytes (Fig. 2). Blood chemical analysis showed a mild increase in total LDH activity. LDH isoenzyme analysis also showed increases in both LDH-2 and -3 fractions (575 and 514 U/L, respectively). Serum thymidine kinase (TK) activity was 527 U/L, which was significantly higher than the reference (< 5.4 U/L, Sakamoto et al. 2009).
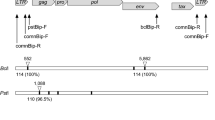
The BLV proviral load was quantified using the Cycleave PCR bovine leukemia virus detection kit (TaKaRa, Shiga, Japan) on the ABI Step One system (Applied Biosystems, CA, USA) according to the manufacturers' instructions. Briefly, pol gene of BLV was amplified and detected with the FAM-labeled Cycleave probe supplied by the kit. Finally, the number of copies of provirus per 10 ng of genomic DNA was calculated. This revealed a markedly higher quantity, at 464 copies/10 ng DNA. Clonality analysis was performed by PCR based on the gene rearrangement of IGH (Maezawa et al. 2020). Using a primer set (BoVHF1: 5′-AGC CCT GAA ATC CCG GCT CA-3′ / BoVHR1: 5′-TCC AGG AGT CCT TGG CCC CA-3′) to amplify the IGH variable region, peripheral blood DNA was used as a template. PCR was performed as previously described (Maezawa et al. 2020) and the products were analyzed by both 2% agarose gel electrophoresis and capillary electrophoresis (Qsep 1-Lite Bio-Fragment Analyzer: Bioptic Inc., Taiwan). A clear single band was recorded with both methods of electrophoresis (Fig. 3), which suggested monoclonal proliferation of peripheral blood B lymphocytes. These findings strongly indicated the presence of a B lymphocyte tumor related to BLV infection, which was interpreted as a sign of EBL.
Necropsy was performed after euthanasia on day 7. No swelling of surface lymph nodes was observed. In the pelvic cavity, a milky white mass measuring 32 × 25 × 8 cm was observed from the distal vagina to the ventral side of the uterus (Fig. 4A). The cut surface of the mass was milky white, pulp-like, and partly necrotic (Fig. 4B). In the abdominal cavity, three masses measuring approximately 8 × 5 × 5 cm were observed in the pyloric region of the abomasum and submucosa of the lesser curvature of the stomach. In the thoracic cavity, multiple milky white masses roughly 1 cm in diameter were observed in the auricle of the right atrium, and milky white foci were also found diffusely in the ventricular muscle.
Histopathological examination revealed that the uterine mass consisted of diffuse proliferation of medium-to-large independent round cells with cellular atypia. Based on these findings, a diagnosis of lymphoma was made. Immunohistochemically, the proliferating neoplastic lymphocytes were negative for the T lymphocyte surface marker CD3 and positive for B lymphocyte surface markers including CD20 and BLA36 (Fig. 5), suggesting B cell lymphoma.
Histopathological features of the uterine mass. The uterine mass showed diffuse proliferation of medium-to-large independent round cells with cellular atypia (A) and the diagnosis of lymphoma was made accordingly. HE staining (A) immunohistochemistry of enlarged lymph nodes revealed neoplastic lymphocytes positive for CD20 (B) and BLA36 (C), but negative for CD3 (D), suggestive of B cell lymphoma. Immunohistochemistry with hematoxylin counter stain (B–D). Bar = 50 µm
Discussion
In the present case, the local veterinarian first suspected EBL even in the absence of lymphadenopathy or lymphocytosis, because the patient showed clinical signs of anorexia, the antibody against BLV was positive, and a mass was palpable on the uterine wall by rectal examination. Following patient transfer to the university hospital for definitive diagnosis, the peripheral blood smear showed approximately 10% of atypical lymphocytes with abnormal nuclei, including constricted or flower-like morphology. However, further evidence was needed in order to determine whether these were lymphoma cells or not. This is because cattle occasionally are presented with atypical lymphocytes in the peripheral blood (so-called ‘reactive lymphocytes’), which lead to inflammation as a pathogenic response (Harvey 2012). It is exceedingly difficult to distinguish microscopically between abnormal lymphocytes (tumor cells) and reactive lymphocytes.
In recent years, clonality analysis of lymphocyte subpopulation based on the rearrangement of immunoglobulin genes for B cells and T cell receptor genes for T cells, respectively, has been used for diagnosis of lymphoma/leukemia in humans and small animals (Frouzi et al. 2017; Hwang et al. 2019). Clonality analysis of bovine B cells based on IGH gene rearrangement has also been reported (Maezawa et al. 2020; Nishimori et al. 2017). Thus, clonality analysis was applied in the present case as an additional test to prove monoclonal proliferation of B cells in peripheral blood. The result strongly suggested that the atypical lymphocytes found in the peripheral blood were due to neoplastic proliferation of B cells or tumorigenic ‘abnormal lymphocytes,’ leading to a definitive diagnosis of B cell lymphoma. In addition, a high BLV provirus load was detected, suggesting that the lymphoma found in the present case was related to BLV infection. Increased serum TK activity, an onset marker for lymphoma (Tawfeeq et al. 2013), also supported the diagnosis of lymphoma.
Sensitivity of PCR for B cell clonality analysis used in this study is reported to be approximately 70% (Maezawa et al. 2020). In other words, this method has a 30% chance of missing B cell lymphoma. Another PCR method targeting other regions of the IGH gene has been reported (Nishimori et al. 2017). Inverse PCR, which detects monoclonal integration of BLV in the patient genome, is also available (Maezawa et al. 2021). We expect that the diagnostic accuracy of B cell lymphoma will be improved by combining several methods to demonstrate clonality.
In conclusion, this is the first clinical case of EBL in the absence of lymphadenopathy and lymphocytosis which was diagnosed by demonstrating monoclonal proliferation of peripheral blood B cells using PCR based on IGH gene rearrangement.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
Code availability
Not applicable.
References
Angelos JA, Thurmond MC (2015) Large animal internal medicine, 5th ed. (Smith, BP. ed.), Elsevier, St. Louis
Constable PD, Hinchcliff KW, Done SH (2017) Veterinary medicine, a textbook of the diseases of cattle, sheep, pigs, goats and horses, 11th Elsevier, St. Louis
Frouzi S, Farmanbar A, Nakai K, Iwanaga M, Uchimaru K, Utsunomiya A, Suzuki Y, Watanabe T (2017) Clonality of HTLV-1–infected T cells as a risk indicator for development and progression of adult T-cell leukemia. Blood Adv 1:1195–1205. https://doi.org/10.1182/bloodadvances.2017005900
George JW, Snipes J, Lane M (2010) Comparison of bovine hematology references intervals from 1957–2006. Vet Clin Pathol 39:138–148
Harvey JW (2012) Veterinary hematology, a diagnostic guide and color atlas. Elsevier Saunders, St. Louis
Hwang M-H, Darzentas N, Moore PF, Guscette F, Morrison J, Keller SM (2019) A review of canine B cell clonality assays and primer set optimization using large-scale repertoire data. Vet Immunol Immunopath 209:45–52. https://doi.org/10.1016/j.vetimm.2019.01.002
Kaneko JJ, Harvey JW, Bruss ML (2008) Proteins, proteomics and the dysproteinemias. Clinical biochemistry of domestic animals, 6th edn. Elsevier, Burlington, pp 117–155
Maezawa M, Sakaguchi K, Tanaka Y, Watanabe K, Kobayashi Y, Inokuma H (2021) Detection of monoclonal or oligoclonal integration of bovine leukemia virus proviral DNA by inverse polymerase chain reaction for diagnosis of enzootic bovine leukosis. Comp Clin Pathol 30:711–714. https://link.springer.com/content/pdf/10.1007/s00580-021-03265-6.pdf
Maezawa M, Watanabe K, Horiuchi N, Matsumoto K, Kobayashi Y, Inokuma H (2020) Molecular diagnosis of bovine B-cell lymphoma using PCR for immunoglobulin heavy chain gene. J Vet Med Sci 82:61–63. https://doi.org/10.1292/jvms.19-0418
Nishimori A, Konnai S, Okagawa T, Maekawa N, Goto S, Ikebuchi R, Nakahara A, Chiba Y, Ikeda M, Murata S, Ohashi K (2017) Identification of an atypical enzootic bovine leukosis in Japan by using a novel classification of bovine leukemia based on immunophenotypic analysis. Clin Vaccine Immunol 24:e00067-e117. https://doi.org/10.1128/CVI.00067-17
Sakamoto L, Obayashi T, Matsumoto K, Kobayashi Y, Inokuma H (2009) Serum thymidine kinase activity as a useful marker for bovine leukosi. J Vet Diag Invest 21:871–874
Tawfeeq MM, Miura S, Kobayashi Y, Furuoka H, Inokuma H (2013) Utility of serum thymidine kinase activity measurements for cases of bovine leukosis with difficult clinical diagnoses. J Vet Med Sci 75:1213–1217. https://doi.org/10.1292/jvms.12-0572
Acknowledgements
Authors thank Dr. Aya Kanzawa for introducing this valuable clinical case of EBL. We also thank all staff members at both the Laboratory of Veterinary Internal Medicine and the Laboratory of Veterinary Pathology at Obihiro University of Agriculture and Veterinary Medicine for their technical help.
Funding
Open access funding provided by The University of Tokyo.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and physical examination of this case were performed by M. Maezawa and H. Inokuma, at Obihiro, Hokkaido. Laboratory analysis, including B lymphocyte clonality test and real-time PCR for BLV, was done by T. Nagata at the University of Tokyo. Necropsy was performed by H. Inokuma, M. Maezawa, K. Watanabe, and Y. Kobayashi at Obihiro University of Agriculture and Veterinary Medicine. Histopathological examination were performed by K. Kojima, J.K. Chambers, and K. Uchida at the University of Tokyo. The first draft of the manuscript was written by H. Inokuma and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Funding
This work was supported by JSPS KAKENHI Grant Numbers 20H03142 and 20J10567.
Conflict of interest
The Laboratory of OSG Veterinary Science for Global Disease Management is an endowment laboratory, supported by a grant from OSG Corporation.
Ethical approval
This study was approved by Obihiro University of Agriculture and Veterinary Medicine Committee for Experiments Using Animals (Approval number: 18–32) based on the Fundamental Guidelines for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions.
Informed consent
For this type of study informed consent is not required.
Consent for publication
For this type of study consent for publication is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Inokuma, H., Nagata, T., Maezawa, M. et al. A clinical case of enzootic bovine leukosis diagnosed by using clonal analysis of peripheral B lymphocytes in a Japanese Black cow. Comp Clin Pathol 32, 195–200 (2023). https://doi.org/10.1007/s00580-022-03432-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-022-03432-3